Abstract
Sufficiency of vitamin K, as K2 specifically, is gaining recognition as a requirement for long term health in many more areas of human physiology than previously realized. The breaking research is revealing a number of roles for vitamin K reaching well beyond coagulation to not only long term cardiovascular and skeletal health, but that of the brain and nervous system, and also for insulin production and sensitivity, and genomic stability / cancer prevention.
An increasing number of aging-associated diseases are now recognized to be related to vitamin K2 insufficiency. Vitamin K1 is not commonly deficient: the vitamin K–dependent (VKD) clotting factors, which are carboxylated (activated) by K1 in the liver, have been found to be fully carboxylated in the healthy population. However, evidence indicates that vitamin K2 is commonly deficient; even in all healthy humans tested, a significant percentage of the extra-hepatic VKD proteins, which are activated by vitamin K2, e.g., osteocalcin and matrix Gla-protein, are only partly carboxylated and therefore inactive. Increasing vitamin K intake has been shown to increase carboxylation of extra-hepatic VKD proteins in apparently healthy adults, as well as those with osteoporosis and atherosclerosis. This indicates that current intake (and RDA) values for vitamin K are too low to support proper function of extra-hepatic Gla-proteins. Foods containing K2 in the Western diet are concentrated sources of calories, cholesterol and saturated fat, thus supplementation is advisable.
As a supplement, vitamin K is available in its K1, MK-4 and MK-7 forms. Until recently, the majority of the research investigating vitamin K’s potential for the prevention and treatment of age-associated diseases has been conducted using pharmacological doses of supplemental K1 or MK-4. However, the latest studies indicate that much smaller doses of MK-7 in combination with K1—both in amounts potentially derived from the diet—may be preferable for a number of reasons. These topics are the primary focus of this article following a brief summary of the mechanism(s) of action through which vitamin K exerts its key protective functions; an examination of why needs for vitamin K (both as K1 and K2), and other micronutrients, increase with age; and a review of the expanding list of age-associated diseases for which K2 insufficiency is being revealed to be an important contributing factor.
Introduction
Similar to “Vitamin E,” “Vitamin K” is not a single substance, but a family of structurally similar, fat-soluble compounds that share a naphthoquinone ring structure with a methyl group at position 2 and an aliphatic side chain at position 3. In nature, vitamin K appears primarily in two forms: phylloquinone (K1), which has an aliphatic side chain of four prenyl residues, the first of which is unsaturated, and the menaquinones (K2), in which the number of prenyl residues may vary from 4 to 13, and all are unsaturated.1 (K3, the 2-methylnaphthoquinone ring without a side chain, is a synthetic analogue used to potentiate cancer chemotherapy and is banned from use in nutritional supplements. 2
Primary Forms of Vitamin K 3
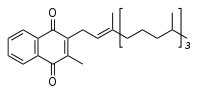 Vitamin K1 (phylloquinone). Both the K1 and K2 forms of the vitamin contain a functional napthoquinone ring and an aliphatic side chain. Phylloquinone has a phytyl side chain.
Vitamin K1 (phylloquinone). Both the K1 and K2 forms of the vitamin contain a functional napthoquinone ring and an aliphatic side chain. Phylloquinone has a phytyl side chain.
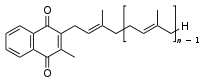 Vitamin K2 (menaquinone). In menaquinone, the side chain is composed of a varying number of isoprenoid residues.
Vitamin K2 (menaquinone). In menaquinone, the side chain is composed of a varying number of isoprenoid residues.
Vitamin K1, a single compound of plant origin found in green leafy vegetables (notably kale, spinach, broccoli, and Brussels sprouts, thus its Latin name “phylloquinone” [phylo = leaf]) and some vegetable oils [e.g., rapeseed, olive, soy, canola oils]), is the primary form of vitamin K in the Western diet.
Vitamin K2, of bacterial origin, refers to the series of menaquinones that share a common methylated napthoquinone ring structure (thus their mena prefix), but differ in the length and degree of saturation of their aliphatic side chain. In humans, the most common menaquinones (MK) are the short-chain MK-4, which is produced endogenously via systemic conversion of K1 to MK-4, and the long-chain MKs, MK-7 through MK-10, which are synthesized by intestinal bacteria in all mammals, including humans. Both MK-4 and the long-chain MKs are present in the diet in minute (mcg) amounts (see Table 1: Micrograms of Vitamin K Present in Foods). Different bacteria synthesize side chains varying from 6 to 11 prenyl units (MK-6 through MK-11); the 4 unit menaquinone (MK-4) is, at most, a minor bacterial product. Nutritionally significant amounts of long-chain MKs also occur in the diet in a few fermented foods. 1
The richest food source of K2 is the Japanese fermented soy bean food, natto, which is produced with Bacillus natto, a bacterium that converts K1 to MK-7. Outside Japan, cheese, which contains MK-8 and MK-9 and is another food produced with the help of bacteria, is the most important dietary source of K2. Proprioni bacteria, for example, which ferment Swiss Emmental and Norwegian Jarlsberg cheeses, produce MK-9. However, the concentration of K2 in cheese is 20 to 40-fold lower than that in natto. In the U.S., meats and eggs are the most common sources of K2, in the form of MK-4; the concentration of K2 in meat and eggs is >40-fold lower than that in natto. 5, 6, 7
Dietary intake of vitamin K is much higher in the Netherlands than the U.S. (250 mcg versus 80 mcg/day), presumably because of much higher vegetable consumption by the Dutch. In the Rotterdam study, dietary vitamin K intakes corresponding to the highest quartile were 370 mcg/day for K1 and 45 mcg/day of menaquinones (MK-7, MK-8, MK-9), which corresponds to consumption of 100 grams (~3 ounces) green vegetables and 100g (~ 3 oz) of cheese, respectively.
| Micrograms of Vitamin K Present in Foods (mcg in 100 g or 100 ml)* | |||
| Food | K1 | MK-4 | MK-7,8,9 |
| Meats | 0.5-5 | 1-30 | 0.1-2 |
| Fish | 0.1-1 | 0.1-2 | |
| Green vegs | 100-750 | ||
| Natto | 20-40 | 900-1200 | |
| Cheese | 0.5-10 | 0.5-10 | 40-80 |
| Other dairy | 0.5 15 | 0.2-15 | 0-35 |
| Eggs | 0.5-2.5 | 10-25 | |
*Adapted from Schurgers LJ, Geleijnse JM, Grobbee DE, et al. Nutritional intake of vitamins K1 (phylloquinone) and K2 (menaquinone) in the Netherlands. J. Nutr. Environ. Med. June 1999;9(2):115–122. DOI: 10.1080/13590849961717
Vitamin K Protects against Degenerative Diseases of Aging by Activating Vitamin K-Dependent Proteins
Vitamin K plays an essential role in the prevention of degenerative age-associated disease due to its being the required cofactor for the enzyme, γ-glutamyl carboxylase, which converts protein-bound glutamate residues (Glu) into γ-carboxyglutamate (Gla) residues in the Gla- (aka vitamin K-dependent [VKD]) proteins. Their Gla-residues form the calcium-binding sites necessary for the biologic activity of these proteins. In the case of vitamin K insufficiency, the carboxylation reaction cannot proceed, and Gla-proteins are released into the circulation in an undercarboxylated form. Undercarboxylated Gla-proteins (also referred to in the literature as Glu-proteins) are inactive.
Fully carboxylated Gla-proteins are involved in numerous critical activities throughout the body, including blood coagulation (K1); bone metabolism, vascular repair, prevention of vascular calcification, cell division and proliferation, myelin development and signal transduction; insulin sensitivity and glucose metabolism (K2). K1’s anti-inflammatory actions also suggest a protective role for this form of vitamin K against age-related chronic diseases including osteoporosis, cardiovascular diseases, type 2 diabetes, osteoarthritis and Alzheimer’s disease. Key recent studies exploring these actions of vitamin K are discussed below under the subheading Vitamin K: A Key Player in the Longevity Game.
Vitamin K Needs in Apparently Healthy Humans Unmet for Extra-Hepatic Vitamin K-Dependent Proteins
Vitamin K1 deficiency, except in newborns, is virtually unknown. The vitamin K–dependent (VKD) clotting factors are all produced in the liver, and all are fully carboxylated (activated by vitamin K1) in the healthy population. 9 However, evidence suggests that vitamin K2 is a very common deficiency: in all apparently healthy humans tested, the extra-hepatic VKD (aka Gla-) proteins, osteocalcin and matrix Gla-protein, have been found to be only partly carboxylated (activated by vitamin K2). About 30% of the circulating osteocalcin (OC) occurs in its undercarboxylated form (ucOC) in healthy adults, and this percentage increases with osteopenia / osteoporosis.10, 11, 12 An analogous percentage of uncarboxylated matrix-Gla protein (ucMGP) is seen in healthy, and progressively unhealthy arteries. In arteries devoid of calcification and lipid or macrophage infiltration, ~30% of the MGP is uncarboxylated. 13 As lipid infiltration and inflammation increase, ucMGP not only accumulates in vesicular structures, but contributes to vessel wall calcification. In Stage III atherosclerosis, expression of ucMGP accelerates in the vessel wall, overwhelming the VKD–dependent carboxylation machinery due to lack of K2 reserves.9, 14 Increasing vitamin K intake has been shown to increase carboxylation of both OC and MGP in apparently healthy adults, as well as in adults with osteoporosis and atherosclerosis. This indicates that current intake (and RDA) values for vitamin K are too low to provide full carboxylation (and thus proper function) of extra-hepatic Gla-proteins.
Since the foods in the Western diet that deliver MKs – dairy, eggs and meat — are concentrated sources of calories, cholesterol and saturated fat, K2 supplementation is advisable to promote full carboxylation of the VKD proteins required to prevent the increasingly long list of aging-associated diseases now recognized to be related to vitamin K2 insufficiency. 13, 15 As a supplement, vitamin K2 is available in its MK-4 and MK-7 forms: MK-4 as a synthetic version (menatetrenone), and MK-7 as the natural compound extracted from natto. Until recently, the majority of the research investigating vitamin K’s potential for the prevention and treatment of age-associated diseases (specifically, cardiovascular disease and osteoporosis) has been conducted using pharmacological doses of supplemental K1 (1-5 mg/day) or MK-4 (15 mg t.i.d., i.e., 45 mg/day). For a review of the principal studies, please see our earlier article, Vitamin D and Vitamin K Team Up to Lower CVD Risk, Part II: The Vitamin K Connection to Cardiovascular Health. However, the latest studies indicate that much smaller doses of MK-7 in combination with K1—both in amounts potentially derived from the diet—may be preferable for a number of reasons. These will be a primary focus of this article following a brief recap of the mechanism(s) of action through which vitamin K exerts its key protective functions; a discussion of why needs for vitamin K (both as K1 and K2), and other micronutrients, increase with age; and a review of the expanding list of age-associated diseases for which K2 insufficiency is being revealed to be an important contributing factor.
Vitamin K: A Key Player in the Longevity Game
Sufficiency of vitamin K, as K2 specifically, is gaining recognition as a requirement for long term health in many more areas of human physiology than previously realized. The breaking research is revealing a number of roles for vitamin K reaching well beyond coagulation to not only long term cardiovascular and skeletal health, but also that of the brain and nervous system, insulin production and sensitivity, and genomic stability / cancer prevention.
K2’s Regulation of the Body’s Use of Calcium Critical for Bone, Vascular and Kidney Health
Seventeen Gla-proteins have been identified, 7 of which are carboxylated by K1 in the liver, where K1 is preferentially utilized to carboxylate the Gla-proteins involved in regulating blood coagulation. The 10 Gla-proteins not synthesized in the liver (aka, the extra-hepatic VKD or Gla-proteins) are carboxylated by K2, and include two key regulators of calcium usage: osteocalcin (OC), which is primarily synthesized in, and required for calcium deposition in, bone, and plays a key role in the prevention of osteoporosis; and matrix Gla-protein, (MGP), which is primarily synthesized in cartilage and the vessel wall, and prevents calcium deposition in the vasculature and myocardium. Activated MGP, the strongest inhibitor of tissue calcification presently known, is essential for the prevention of coronary artery disease. 16, 17
In addition to osteocalcin and MGP, another recently discovered extra-hepatic, calcium-regulatory Gla-protein, the Gla-rich protein (GRP) — is a circulating protein expressed and accumulated not only in cartilage and bone, but also in soft tissues, particularly the vascular system and skin. Gla-rich protein, which is just being investigated, appears to have the highest content of Gla residues of all the Gla-proteins, and thus an uncommonly high ability to bind calcium. High concentratioins of ucGRP have recently been identified at sites of pathological calcification in the skin (e.g., in pseudoxanthoma elasticum (PXE),an autosomal recessive disorder in which calcification of connective tissue leads to pathology in skin, eye and blood vessels; and dermatomyositis with calcinosis, a rare, often chronic autoimmune disease with onset during childhood that is characterized by weakness in proximal muscles and pathognomonic skin rashes), vasculature (arterial calcification) and kidneys (chronic kidney disease). 18
Vitamin K Insufficiency – A Risk Factor for Kidney Disease?
It has been repeatedly demonstrated that vascular calcification is present in patients suffering from advanced chronic kidney disease (CKD) to a far greater extent than in individuals with normal renal function; 50% of mortalities in patients with CKD undergoing dialysis treatment are related to vascular calcification. 19, 20 On the other hand, 20% to 40% of the patients in most CKD cohorts do not develop detectable vascular calcification despite exposure to well-known environmental triggers, such as uremia, diabetes, and hyperphosphatemia. This suggests that naturally occurring vascular calcification inhibitors (i.e., MGP — after activation by posttranslational γ-glutamyl carboxylation of its five glutamate residues, which requires K2) play an important role in preventing this disease process. 21
A recent study evaluating the association between circulating inactive MGP and all-cause mortality in 188 hemodialysis patients, compared with 98 age-matched subjects with normal renal function, supports this hypothesis. Even low levels of inactive MGP were found to increase all-cause mortality risk by 220% and risk of death from cardiovascular disease by 270%. Furthermore, patients with higher vascular calcification scores showed lower levels of inactive MGP. In 17 hemodialysis patients, daily supplementation with vitamin K2 for 6 weeks reduced ucMGP levels by 27%. 21
Patients with CKD are also now being recognized to have greatly increased susceptibility to fragility fracture as well as vascular calcifications, both of which are known to be related to vitamin K insufficiency. 22 These findings indicate that vitamin K insufficiency (particularly of K2, the form in which vitamin K activates extra-hepatic Gla-proteins) is an important contributing factor to the dysregulation of calcium usage that contributes to both lack of appropriate calcification in bone and calcium’s pathological deposition in the vasculature, including in the kidneys, where it is a key factor in the development and progression of chronic kidney disease.
Coumarins (vitamin K antagonists used as coagulation inhibitors) are now well known to be a risk factor for vascular calcification. In recently published studies, patients on coumarins have been found to have significantly higher levels of uncarboxylated /inactive MGP (ucMGP); plasma levels of ucMGP have been shown to increase significantly with the use of vitamin K antagonists and, conversely, to decrease after supplementation with vitamin K. 23
K2 Supplementation Essential in Patients Supplementing with Calcium and Vitamin D
Vitamin K2 is essential to prevent adverse coronary and kidney outcomes in patients supplementing with calcium to prevent osteoporosis, especially if these patients are also being given vitamin D.
Two studies published April 2011 underscore the importance to patient outcomes of an awareness of K2’s role in regulating calcium deposition, and the requirement for K2 sufficient to balance the increased absorption of calcium that occurs when supplemental vitamin D is given. Both studies reported significant increased risk of adverse outcomes in women taking calcium supplements with or without vitamin D.
The first study, widely broadcast in the news after it appeared in the British Medical Journal, was a seven year, randomized, placebo-controlled trial of daily supplementation with calcium (1 gram) and vitamin D (400 IU) in 36,282 postmenopausal women in the Women’s Health Initiative (WHI) study. Meta-analysis of three placebo-controlled trials found calcium and vitamin D increased risk of myocardial infarction 24% and the composite of myocardial infarction or stroke 15%. The conclusion drawn by Bolland et al.: “A reassessment of the role of calcium supplements in osteoporosis management is warranted.” 24
The second paper, published in the American Journal of Clinical Nutrition analyzed data collected on the same 36,282 postmenopausal women participating in the WHI, this time in relation to whether calcium plus vitamin D supplements increased risk of kidney stone formation. Unsurprisingly, a 17% excess in urinary tract stone incidence was noted in the women taking both supplements. 25
Unfortunately, neither in the articles reporting these two studies, nor in the press feeding frenzy that occurred after their publication, was vitamin K’s role in regulating calcium even considered. Had it been, these highly negative outcomes could have easily been predicted – and more importantly, avoided.

Safe D by NutriCrafters: Vitamin D3 combined with vitamin K2 in the natural MK7 form. Each bottle contains 1,500 drops and each drop contains 160 iu (international units) of vitamin D3 and 1.8 mcg Vitamin K2.
It is well known that vitamin D boosts calcium’s absorption from the intestines and its re-absorption from the kidneys, thus greatly enhancing levels of available calcium within the body. Less widely known is that Vitamin D upregulates the expression of Gla-proteins, whose activation depends on vitamin K-mediated carboxylation. Vitamin D thus increases both the demand for vitamin K and the potential for benefit from K-dependent proteins, including osteocalcin in bone and MGP in blood vessels. 26
As noted in Part II of our 2009 LMR review, Vitamin D and Vitamin K Team Up to Lower CVD Risk, one potentially adverse repercussion of this, is that by increasing the need for vitamin K2, increased levels of vitamin D may actually induce a functional vitamin K2 deficiency, with the result that levels of ucOC and ucMGP rise in the circulation and vasculature. In this case, not only is calcium not delivered to the bones, which become porous, but it is deposited in the arteries, which become calcified, and also overloads the kidneys, promoting stone formation. 27
Vitamin D toxicity has been proposed to be the result of precisely such induction of vitamin K2 deficiency. 27 As vitamin D induces levels of Gla proteins to rise, the pool of available vitamin K available to carboxylate them becomes depleted, so vitamin K-dependent processes that retain minerals in the bone matrix, and protect the soft tissues from calcification, can no longer be performed. That warfarin, a coumadin derivative that induces a functional vitamin K deficiency, has definitively been shown to produce extensive hypervitaminosis D-like calcification of the soft tissues, and to exert toxicity synergistically with vitamin D when the two are combined, supports this hypothesis. 28 In addition, vitamin K alone has been shown to fully reverse the calcification induced by warfarin, both confirming that the drug’s inhibition of vitamin K is directly responsible for its induction of calcification, and also adding to the likelihood that vitamin D toxicity is due to the same or a similar mechanism. 29
Vitamin K Insufficiency – A Risk Factor for Alzheimer’s Disease?
Recent studies have indicated an association between Alzheimer’s disease (AD) and vitamin K insufficiency. In 2005, a cross-sectional study of 100 Japanese women with AD (mean age, 79.8 years) and 100 age-matched community dwelling controls (mean age, 80.6 years), reported bone mineral density and serum concentrations of K1 to be significantly lower, and serum levels of ucOC significantly higher, in severely demented patients than in those with mild dementia. 30
In 2008, AD patients’ poor K1 status was confirmed with the publication of a study looking at associations between vitamin K status and BMD in AD patients in the Netherlands. AD patients’ dietary intake of K1 was found to be less than 50% of that in age-matched controls, and patients in the early stages of probable AD had significantly lower vitamin K intakes than age- and sex-matched healthy participants, a difference that remained highly significant after data were adjusted for energy intakes. The authors suggested that vitamin K insufficiency could not only be a risk factor for AD, but could also contribute to its accelerated progression. 31
Other research has shown that warfarin, a coumarin derivative that prevents vitamin K recycling, leads to increased β-amyloid deposition in brain vessels. 32 (During gamma-glutamate carboxylation, vitamin K is oxidized into its epoxide form (KO), which is reconverted to vitamin K quinone (K) by the enzyme vitamin K epoxide reductase (VKOR). Derivatives of 4-hydroxycoumarin (including warfarin and acenocoumarol) specifically inhibit VKOR, thus preventing the recycling of vitamin K.) 33
In a paper published in Medical Hypotheses in 2001, Allison proposed that Alzheimer’s disease (AD) is associated with vitamin K status, based on the observation that the circulating concentration of K1 is significantly lower, and incidence of AD significantly higher, in ApoE4 carriers than in those with other ApoE genotypes. 1
This inverse relationship results from the fact that vitamin K in plasma is bound to chylomicrons and chylomicron remnants, which carry apolipoprotein E. Clearance of chylomicrons and their remnants from the circulation depends on ApoE’s binding to a hepatic receptor, and the rate at which this binding takes place is greatly influenced by ApoE genotype, occurring rapidly in people carrying one or two alleles for E4 and slowly in those whose genes encode E2. Thus ApoE genotype significantly impacts blood levels of vitamin K, which are highest in E2, intermediate in E3 and lowest in E4.
Not surprisingly, carriers of ApoE4 have been found to have significantly higher levels of undercarboxylated osteocalcin (ucOC), indicating extra-hepatic vitamin K deficiency. In confirmation of ApoE4’s deleterious effects on bone, women ≥ 65 years who carry the E4 allele are known to have a significantly increased risk of osteoporotic hip fractures compared with those bearing E2 or E3. 34, 35
ApoE4 carriers are also at greatly increased risk for brain atrophy, and this risk is compounded by cerebrovascular disease. Analysis of quantitative magnetic resonance imaging (MRI) findings on 396 surviving members of the National Heart, Lung and Blood Institute Twin study revealed that carriers of ApoE4 had significantly smaller brain volumes than those with other ApoE genotypes. The presence of both cerebrovascular disease and ApoE4 was associated with significantly greater brain atrophy than was seen with ApoE4 or cerebrovascular disease alone. The authors concluded that ApoE4 enhances the extent of brain degeneration by increasing the brain’s susceptibility to injury and/or impairing brain repair mechanisms.
A possible mechanism for ApoE4’s deleterious effects on the brain is insufficiency of vitamin K, a blood borne factor whose concentration is lowered by ApoE4, and whose actions in the brain improve neuron survival and repair after brain injury.
Vitamin K’s Neuroprotective Activities
That vitamin K, found in high concentrations in the brain as MK-4, is required for normal brain development and function has been demonstrated by that fact that maternal exposure to coumarin derivatives, particularly in the second trimester of pregnancy, causes central nervous system abnormalities and mental retardation in offspring. 36, 37
In the adult brain, vitamin K2 remains involved in sulfation, which is decreased in AD
K2 is required for the activity of brain galactocerebroside sulfotransferase (GST), an enzyme involved in the production of neuronal myelin sulfatides (the development of myelin involves sulfatide accumulation); brain sphingolipid metabolism; and the production of keratin sulfate (KS), levels of which are dramatically reduced in the cerebral cortex of AD patients compared to age-matched AD-free controls.
The KS are sulfated proteoglycans found within several cell types and on their surfaces, where they are components of adhesion molecules. A key KS, SV-2, is a major protein of synaptic vesicles, is structurally similar to neurotransmitter transporters, and is involved in acetylcholine transport. SV-2 is present in synapses in the normal brain, but lacking in cortical neurons in AD, consistent with the loss of synaptic function seen in AD.
K2 protects against neuronal apoptosis, which is increased in AD
Gas-6, another VKD-protein in the brain, is a product of growth arrest specific gene 6, and along with its tyrosine kinase receptor, Ark, is widely distributed throughout the central nervous system. Interaction of Gas-6 with Ark plays a protective role in the brain, both as a growth factor for Schwann cells (non-neuronal cells that maintain homeostasis, wrap around axons of motor and sensory neurons to form the myelin sheath, and provide support and protection for the brain’s neurons) and by preventing neurons from entering apoptosis. That the VKD Gas-6-tyrosine kinase receptor can protect neurons against apoptosis is obviously relevant to AD, in which neuronal apoptosis plays a significant role.
Hydrogenation of Oils Promotes Functional Vitamin K Deficiency
Of note, the human body converts K1 to MK-4 in the brain—the form in which vitamin K is involved in brain sulfation. However, humans are unable to convert the hydrogenated K1 found in hydrogenated vegetable oils to MK-4 either in the brain or elsewhere. 38
This deleterious effect of hydrogenation is particularly relevant in the U.S. since, as noted in our earlier article, Vitamin D and Vitamin K Team Up to Lower CVD Risk, Part II: The Vitamin K Connection to Cardiovascular Health, canola and soybean oils are the primary source of vitamin K in the American diet. Hydrogenation changes the K1 (phylloquinone) in these oils into dihydrophylloquinone, a form incapable of carboxylating VKD proteins. Since osteocalcin is also VKD-protein, one would expect bone health in aging adults to be among the long-term adverse outcomes of hydrogenation, and data from the Framingham Offspring Study (n=2,544, average age 58.5) confirm this hypothesis. Subjects with the highest intake of vitamin K1 from hydrogenated oils, the type of oil typically used in processed and fast foods, had the lowest BMD at the neck, hip and spine. 39 The clinical take away: the Standard American Diet, in which processed and fast foods containing hydrogenated oils feature significantly, greatly increases risk of functional vitamin K deficiency. 40
Vitamin K2 Insufficiency – A Risk Factor for Type 2 Diabetes?
In addition to a number of animal studies, several recent human studies have suggested an inverse association between vitamin K intake and type-2 diabetes:
Higher dietary K1 intake was associated with greater insulin sensitivity and improved glycemic status in the Framingham Offspring Cohort (~2,000 subjects) 41, and a post hoc analysis of a placebo-controlled trial of K1 supplementation (initially designed to look at K1’s effects on bone health), revealed that insulin resistance was significantly lower in supplemented older men, after three years of follow-up. 42
To date, only one study has compared the effects of K1 versus K2 on type-2 diabetes, and this research looked at dietary, not supplemental vitamin K. A clear inverse association was found for dietary intake of K2, but not K1, which showed only a weak, non-significant trend in relation to type-2 diabetes. 43
That dietary K2, but not K1, was found to be inversely related to type 2 diabetes is particularly intriguing given the primary dietary sources of K1 (leafy greens) and K2 (cheese, in which K2 is present as the longer chain MKs, MK-8 & MK-9), since diets higher in leafy greens are generally considered protective, while those higher in saturated fat and calories are thought to contribute to MetS and insulin resistance. 44 As will be discussed in more detail below, that K2 (in the form of the longer-chain menaquinones, MK-7, MK-8, MK-9), remains systemically available to activate extra-hepatic VKD-proteins for 96 hours (4 days), while K1 is preferentially utilized to carboxylate hepatic clotting proteins, and both K1 and MK-4 are cleared within 8 hours, may help explain this outcome. 5
The Osteocalcin Connection to Insulin Sensitivity
The majority of studies have reported that levels of undercarboxylated osteocalcin (ucOC) increase with older age. (Much inactive circulating osteocalcin is partially carboxylated, but since partial carboxylation still results in its being inactive, this distinction is typically not made, and it is referred to as un– rather than under-carboxylated.) Historically, the primary focus of clinical data on osteocalcin (OC) has been its relationship to skeletal health, for which a consistent finding has been an inverse relationship between levels of ucOC and BMD. High levels of ucOC increase fracture risk; postmenopausal women with high ucOC have been found to have a 6-fold increased risk of hip fracture. 45, 8 (ucOC is known to increase in postmenopausal women and has been negatively correlated with endogenous estradiol levels. Levels of ucOC have been shown to increase after bilateral oophorectomy in premenopausal women, and to decrease with hormone replacement therapy. 46) Most recently, researchers have begun looking into OC’s relationship to energy metabolism.
Diabetes, a disease of low insulin secretion and/or insulin resistance and hyperglycemia, has now been shown to be associated with reduced serum OC levels in rodent models and human studies. The rodent model studies of Karsenty’s group jump-started investigation into OC’s effects on energy metabolism with their 2007 publication in Cell of ‘‘Endocrine regulation of energy metabolism by the skeleton.” In this paper, they were the first to suggest that bone, via osteocalcin, acts as an endocrine organ to influence glucose homeostasis. 47
Karsenty’s initial “ah-ha” came from an observation that mice deficient in OC were a little fat. Further examination revealed that these mice owed their visceral plumpness to a number of glucose metabolism abnormalities: compared to wild type mice, OC-deficient mice had higher blood glucose, lower serum insulin, impaired glucose-stimulated insulin secretion (GSIS) and poor glucose tolerance. The low serum insulin was found to be the result of a near 50% decrease in pancreatic β-cell mass and insulin content. The OC-knockout mice also had reduced levels of serum adiponectin (an adipocytokine that promotes insulin sensitivity), suggesting a role for OC in insulin sensitivity as well as secretion. Karsenty et al’s research revealed that ex vivo (in rodent cell-based assays), ucOC, but not cOC stimulates CyclinD1 (a molecular marker of cell proliferation), insulin expression in β-cells and adiponectin (levels of which are higher in those with better insulin sensitivity and reduced in diabetes); and in vivo, in rodents, ucOC can improve glucose tolerance.
In humans, however, cOC appears to be the form in which OC improves glucose tolerance. 48 In humans, type-1 and type-2 diabetes are associated with lower levels of total (fully carboxylated + partially uncarboxylated) OC. Among those with diabetes, higher total OC is associated with lower fasting glucose and HbA1c (glycosylated hemoglobin). In a study of 50 poorly controlled diabetic patients, Kanazawa et al. reported a decrease in the mean %ucOC with the achievement of improved glycemic control. 49
Perhaps even more interesting, total OC also appears to be associated with glucose metabolism and insulin resistance in those without diabetes. A study in which 149 non-diabetic men were assessed, using the frequently sampled intravenous glucose tolerance test, reported a positive correlation between higher levels of total OC and insulin sensitivity. In a study of older Swedish men, higher levels of OC also negatively correlated with fasting glucose, fasting insulin and HOMA-IR in those without diabetes. 46 (HOMA, the homeostatic model assessment, is a method used to quantify insulin resistance [HOMA-IR].)
The post hoc analysis of the 3-year trial initially designed to determine the effect of vitamin K supplementation on bone loss (noted above) evaluated the effects of vitamin K’s increasing carboxylation of OC (lowering levels of ucOC) on glucose and lipid metabolism. Levels of ucOC dropped in participants (N = 355) randomized to receive 500 mcg/day of K1, compared to the placebo group. Over the 36-months of the study, mean %ucOC dropped ~19% in supplemented men and women. Measurements of fasting plasma glucose and insulin, and calculated HOMA-IR, were compared at the baseline, 6-month and 36 month visits. At 36 months, men in the vitamin K-treated group, but not the women, showed improved insulin sensitivity based on HOMA-IR, compared with placebo.42 Regarding the lack of effect on insulin sensitivity in women, Motyl et al. suggest that these were healthy women, so changes in ucOC with vitamin K supplementation may have been too small to produce significant differences in insulin sensitivity over 3 years. 46
In an analysis of the placebo group from this same trial of vitamin K supplementation that reduced ucOC levels, Shea et al. considered whether baseline levels of OC, ucOC or carboxylated (c)OC predicted changes over 3 years of follow-up in HOMA-IR, fasting insulin and fasting glucose. Lower baseline levels of cOC and higher levels of %ucOC were revealed to have accurately predicted greater increases in insulin resistance. 50
In sum, human studies are reporting an association between lower total and %cOC and impaired glucose metabolism and insulin resistance. Diabetes (type-1 and type-2) is associated with lower total OC, and total OC is negatively correlated with HbA1c, fasting glucose and insulin resistance. Lower baseline levels of cOC and higher levels of %ucOC predict greater increases in insulin resistance. Improved glycemic control appears to increase total OC, but not ucOC. 51, 52, 53, 46
Current thinking is that the acidic conditions (low pH) created by the osteoclastic resorption process activate osteocalcin inside the bone, which is then released from bone to stimulate pancreatic insulin secretion. 54 Additionally, it has very recently been discovered that human adipose tissue also produces osteocalcin, adding further complexity to its role in energy metabolism. 55 In contrast to the findings reported in regard to OC’s relationship to insulin sensitiivity, Foresta et al. not only found a lower ucOC/cOC ratio in the overweight and obese patients, but also detected expression of osteocalcin mRNA in subcutaneous and omental adipose tissues (aka, visceral adipose tissue), and documented that adipose tissue releases, in vitro, both ucOCN and cOCN. Further studies are needed to clarify the precise regulation of OC carboxylation and release from adipose tissue in obese vs. normal-weight subjects, taking into account factors that influence adipose tissue function, e.g., inflammatory cytokines.
In their paper in Cell, Karsenty et al. raise the teleological questions: “Why would a bone-specific hormone regulate energy metabolism? What is the need for a hormone favoring β-cell proliferation and insulin secretion?” 47 They speculate that given the large surface covered by the skeleton, it is an excellent site of hormone synthesis, which suggests that other hormones remain to be identified in osteoblasts. Alternatively, OC (and possibly other hormones) may have been recruited to the skeleton through tinkering during evolution, and the pro-proliferation function of OC may have been required during evolution to maintain the constant size of pancreatic islets in periods of food deprivation.
We would like to suggest that OC’s β-cell proliferation and insulin-sensitizing functions help ensure the availability of ATP for the production of bone and its strengthening in response to muscle contractions, an energy-consuming process.
The next steps will be to determine precisely how osteocalcin — as cOC and/or ucOC — interacts with pancreatic β- cells. Are osteocalcin-specific cell surface receptors present on pancreatic β-cells? If so, what are their affinities for cOC and ucOC? Regardless, vitamin K, as both K1 and K2, is highly likely to be found to play an important role.
K1’s Anti-Inflammatory Actions May Promote Insulin Sensitivity
In addition to reducing %ucOC (via its endogenous conversion to MK-4), K1 also reduces inflammation, which may have a separate beneficial effect on insulin sensitivity since inflammation suppresses osteoblast activity and OC expression. 56
In cell culture, animal and human studies, respectively, vitamin K has been shown to: reduce lipopolysaccharide-challenged fibroblasts’ secretion of the pro-inflammatory cytokine IL-6 in vitro; suppress expression of genes involved in an acute inflammatory response in an animal model; and lessen inflammatory responses in certain disease states, as evidenced by significantly lower levels of a number of systemic pro-inflammatory biomarkers in subjects in a Framingham Offspring Study cohort with higher vitamin K plasma concentration or K1 intake. 5
Interestingly, spaceflight, known to cause bone loss via suppression of osteoblast activity and OC expression, also decreases insulin secretion and increases blood glucose levels. 46, 57
MK-7 Likely More Effective in Carboxylating Osteocalcin than K1 or MK-4
MK-7 has been shown to have significantly greater efficacy than K1 in carboxylating OC when taken as a daily supplement ( 0.22 μmol/day), and this is thought to be due to MK-7’s much longer residence time and the higher serum concentrations of MK-7 achieved during its prolonged intake. 9 For the same reasons, MK-7 is likely to be more effective in carboxylating OC than MK-4, since MK-4 and K1 share comparable molecular structures (both contain 4 isoprenoid residues, 3 of which are saturated in K1 but contain a double bond in MK-4) and clearance rates. The longer-chain menaquinones, including MK-7, are much more hydrophobic, are handled differently, and have much longer half-life times (8 hours for K1 and MK-4 versus 96 hours for MK-7). 58
Vitamin K2 Insufficiency – A Risk Factor for Cancer
Other recent studies indicate K2 (as the long chain menaquinones, MK7, MK-8, MK-9; not MK-4) may play a highly protective role against cancer. In 2008, Nimptsch et al. analyzed data from the EPIC-Heidelberg cohort and found that men in the highest quartile of dietary K2 intake (46 mcg/day) had ~50% lower prostate cancer incidence and mortality compared to those in the lowest quartile of K2 intake. No association was found between K1 intake and prostate cancer. As noted above, 5, 6 cheese, which provides the longer-chain menaquinones (MK-8 and MK-9), is the primary source of dietary K2 in this population. 59 Most recently, in a paper published in 2010, Nimptsch et al. extended their analysis of the EPIC-Heidelberg data to other cancers and found all major forms of cancer, except breast cancer, were inversely associated with K2 intake. 60
Two VKD-proteins, Tgfbi and periostin, both of which are involved in maintaining genomic stability during cell division, may help explain K2’s cancer-preventive effects. 61, 62
Earliest Indicator of Vitamin K Insufficiency: Incomplete Carboxylation of Extra-Hepatic VKD-Proteins
As discussed by Ames and McCann in their recent seminal paper proposing nutrient “triage” as a major factor in the development of age-related disease, a key distinction between the hepatic and extra-hepatic Gla-proteins is that the former are fully carboxylated in healthy adults, while the latter are not. “Triage theory” proposes that the reason for this disparity is an evolutionary mechanism via which available nutrients are not equally distributed throughout tissues, but are preempted by those areas in which their use meets the body’s most pressing, immediate survival needs. In relation to vitamin K, triage supports immediate survival by safeguarding against a terminal bleed out, but results in suboptimal vitamin K status in extra-hepatic tissues, which are deprived of the activated VKD proteins required for maintenance of long-term bone and vascular health. 61
After its absorption in the small intestine, where all vitamin K is incorporated into chylomicrons — which form in the small intestine in response to dietary lipid intake, are modified into smaller chylomicron remnants, and rapidly shunted to the liver — vitamin K is efficiently extracted and used to carboxylate the hepatic VKD proteins of the clotting cascade. This “triage effect” promotes subclinical vitamin K insufficiency since only after these Gla-proteins are fully carboxylated is any remaining vitamin K1 converted in the liver to MK-4, incorporated into low-density lipoproteins, and secreted from the liver into the bloodstream for delivery to extra-hepatic tissues*. In contrast, the longer-chain MKs (MK-7,MK-8, MK-9) derived from the diet are preferentially used to activate the extra-hepatic Gla-proteins needed to build bone, prevent arterial calcification, promote the production of myelin and other neuroprotective compounds, and help avert cancer.
*K1 not required for full carboxylation of VKD clotting proteins may also be sent back into the circulation in triglycerides and locally converted into MK-4 via the activity in certain tissues (brain, bone, adipose tissue) of a recently identified prenylation enzyme called UbiA prenyltransferase containing 1 (UBIAD1). 63
Thus, elevated levels of undercarboxylated osteocalcin (ucOC) or MGP (ucMGP), which have been conclusively associated with osteoporosis, and cardiovascular disease and mortality, respectively, serve as early warning indicators of subclinical vitamin K2 deficiency. Since low K2 intake has also been shown to be associated with increased incidence of, and mortality due to, hepatocellular and prostate cancers, elevated levels of two other uncarboxylated Gla-proteins, ucTgfbi and ucPeriostin (Tgfbi, a VKD protein required to maintain genomic stability during mitosis, binds to periostin, another VKD protein, which is therefore also necessary for Tgfbi’s stabilizing effects on the mitotic spindle) may also serve as early warning signals of increased cancer risk due to vitamin K2 insufficiency. 61, 62, 64
Micronutrient Requirements Increase with Age
In addition to triage, aging, in itself, constitutes an indication for increased vitamin requirements. In earlier research discussed in detail in Beyond the Mitochondrial Tune Up: Part I Ames demonstrated that the function of aging mitochondria can be rejuvenated by increasing the availability of nutrients that serve as the substrates or co-factors for key enzymes. This phenomenon is due to the fact that with age, increased oxidative damage to proteins causes increasing, albeit typically slight, deformations in enzymes’ structure, which results in decreased binding affinity for their co-enzyme (i.e., the nutrient co-factor for the enzyme), and thus a decrease in enzyme function.
Another way of stating this is that elders’ enzymes have an increased Michaelis constant/(Km), which is defined as the substrate or co-factor concentration required for half-maximal enzyme activity. The diminished kinetics of enzymes that occur with aging can be counteracted by supplying greater amounts of the co-factor and/or substrate, thus restoring the velocity of enzymes’ activity (Km) — not only in the mitochondria, but, in the case of the VKD Gla-enzymes, in systemic cellular mitosis, bone, the brain and the vasculature.
VKD Proteins Highly Vulnerable to “Kinetics of Aging”
Furthermore, as pointed out by Vermeer and Theuwissen (2011), 65 the VKD proteins are particularly vulnerable to “the kinetics of aging” because vitamin K metabolism is affected by an increased Km requirement for more than one enzyme. Vitamin K acts as both a co-factor for γ-glutamyl carboxylase, and as substrate for DT-diaphorase and vitamin K epoxide reductase (VKOR), the enzymes that recycle vitamin K epoxide into its active hydroquinone form. 33
As noted in Vitamin D and Vitamin K Team Up to Lower CVD Risk, Part II: The Vitamin K Connection to Cardiovascular Health, vitamin K is so critical to survival that the body very efficiently conserves this micronutrient via a cyclic interconversion called the vitamin K cycle. In this recycling process, vitamin K’s quinone form is reduced by the FAD-containing enzyme DT-diaphorase (a.k.a. NAD(P)H:quinone oxidoreductase ) into vitamin K hydroquinone (KH2), which then serves as the cofactor for the carboxylation of Gla-proteins and, in so doing, is oxidized to vitamin K epoxide. Vitamin K epoxide is then recycled back to the quinone form by vitamin K epoxide reductase (VKOR), completing the cycle. On a molecular level, vitamin K expoxide is reduced in two steps: first to the quinone form by VKOR, then to vitamin K hydroquinone (KH2) by DT-diaphorase.
The fact that three enzymes are involved in its metabolism renders vitamin K particularly susceptible to the “kinetics of aging.” This translates into an even greater increase in our requirements for vitamin K to maintain optimal function as we age.
K2, Particularly the Longer Chain Menaquinones, Crucial for Healthy Aging
K2, But Not K1, Carboxylates the VKD-Proteins that Prevent Chronic Age-Associated Disease
Several recently published papers confirm that K2, and specifically the longer-chain menaquinones (MK-7, MK-8, MK-9), but not K1, provide effective protection against cardiovascular disease and osteoporosis.
Gast and de Roos, et al. (2009), 66 examined the relationship between dietary intake of K1 and K2, and K2 subtypes, and CHD incidence in the Prospect-EPIC cohort, which consisted of 16,057 women aged 49-70 years, free of CVD at baseline and followed an average of 8.1 years. Mean vitamin K1 intake was 211.7 +/- 100.3 mcg/d; vitamin K2 intake was 29.1 +/- 12.8 mcg/d. After adjustment for traditional risk and dietary factors, K1 intake was not found to be related to CHD, despite the fact K1 not used for clotting factors in the liver is converted to MK-4; but a significant inverse association was seen between dietary vitamin K2 intake and CHD risk, with a Hazard Ratio (HR) pf 0.91 per 10 mcg/d vitamin K2 intake. Furthermore, and perhaps most importantly, this association was principally due to intake of the longer-chain menaquinones, MK-7, MK-8 and MK-9, primarily supplied by cheese (54%) and milk products (22%) in the Netherlands where this research was conducted. Meat and eggs, which contain MK-4 and MK-7, but primarily MK-4, accounted for only ~15% of dietary intake of K2. Vegetables contributed 82% of vitamin K1 intake.
Other recent study results from the Netherlands show similar inverse associations for dietary intake of the longer-chain menaquinones with coronary calcification. 67 This accumulating evidence helps explain why the inverse association between vitamin K intake and CHD was much less obvious in the two population studies conducted in the U.S., both of which evaluated only K1 and did not collect data on K2. 68, 69 In 2004, the Rotterdam study also reported a strong inverse association between vitamin K2 intake and CHD, but a much less significant association for K1. 15
The fact that the women in the Gast, de Roos et al. 2009 study were postmenopausal renders this subject population of particular relevance for understanding the protective impact of K2 on not only CHD, but osteoporosis since, during the menopausal transition, bone loss accelerates to a rate of 3-10%. A number of other recent studies (discussed in Vitamin D and Vitamin K Team Up to Lower CVD Risk, Part II: The Vitamin K Connection to Cardiovascular Health), also indicate that reduced bone mineral density is a risk factor for CVD.
One cogent example is the MORE study, which included 2,576 postmenopausal women (mean age, 66.5 years. Those with osteoporosis had a 3.9-fold increased risk for cardiovascular events compared to those with low bone mass (BMD); a total hip BMD T score < or = -2.5 versus a T score between -2.5 and -1 was associated with a 2.1-fold increase in risk; and presence of ≥1 vertebral fracture versus no vertebral fracture at baseline was associated with a 3.0-fold increase in risk. Risk of cardiovascular events increased incrementally with the number and increasing severity of baseline vertebral fractures (both p < 0.001). 70
Most recently, Gerber et al. (July 2011) reported a striking association between myocardial infarction (MI) and osteoporotic fractures in a case-controlled study involving 6,642 residents of Olmsted County, Minnesota. Those who had suffered an MI were found to have a 32% increased risk of fracture. 71
As mentioned above, the dietary sources of vitamin K2 (cheese, milk products and meat) differ greatly from those of K1 (leafy greens and unhydrogenated oils) and thus subjects’ higher levels of these forms of vitamin K reflect different dietary patterns. Since a diet rich in K2 contains much more saturated fat (and if cheese is featured, salt, two well-accepted risk factors for cardiovascular disease), than a diet in which K1 predominates, this suggests that the risk reduction in CVD seen with the long-chain MKs, but not K1, is a result of their specific biological effects, i.e., K2 carboxylates extra-hepatic VKD proteins, while hepatic VKD proteins utilize virtually all available K1, with the result that far too little remains available to be sent back into the circulation for intestinal conversion to MK-4 sufficient to meet extra-hepatic Gla-protein needs.
The Body’s Different Uses for K1 and K2 are Demonstrated by Their Different Distributions Over Plasma Lipoproteins
K1 is primarily transported with the triacylglycerol-rich lipoprotein fraction, which is quickly cleared by the liver, promoting its utilization of K1 as a cofactor for the VKD proteins required for blood coagulation and leaving little K1 for use in extra-hepatic tissues. 58
K2 is found in both triacylglycerol-rich lipoprotein and low-density lipoprotein, the latter being its major transport system to extra-hepatic tissues. 58 Animal experiments have confirmed not only that extra-hepatic tissues preferentially accumulate K2, but that K2 (added to the animals’ feed as MK-4 [in pharmacological amounts] in these experiments), but not K1, inhibits warfarin-induced coronary calcification. 29
K1 and MK-4 Share Similar Structure, Thus Both Have a Shorter Half-Life than Long-Chain MKs
As noted above, the longer-chain MKs have a much longer half-life than either K1 or MK-4, which share a very similar molecular structure (both contain 4 isoprenoid residues, 3 of which are saturated in K1 but contain a double bond in MK-4) and therefore similar physiokinetics. Following intestinal absorption, all forms of vitamin K are taken up in the triglyceride fraction from which they are rapidly cleared by the liver, but only the longer-chain menaquinones are redistributed via low-density lipoproteins. This results in both K1 and MK-4 being cleared by the liver within a matter of hours. In contrast, the longer-chain MKs are significantly more lipophilic and are handled differently. Their incorporation into low-density lipoproteins results in their much slower hepatic clearance, several day half-lives, much more stable serum levels and accumulation to 7- to 8-fold higher levels during prolonged intake. 15, 58, 9
In the human population studies discussed above, the K2 subtypes found to be responsible for lowering CHD risk were MK-7, MK-8 and MK-9. The stronger protective effect of the long-chain MKs is thought to be due to their much longer half-life than that of either K1 or MK-4, which renders the long-chain MKs available for carboxylation reactions for a significantly greater time period. 72, 5, 65, 45, 17
Another reason why the long-chain MKs (MK-7, MK-8, MK-9) play a more important physiological role than K1 (and its use for the endogenous production of MK-4) than one might conclude — if looking solely at the relatively lesser amounts of the long-chain MKs present in the diet compared to K1– is that vitamin K’s bioavailability depends not only on intake, but also absorption. K1 is tightly bound to chloroplast membranes in plant cells, from which it is absorbed with low efficiency. After ingestion of a standardized amount of spinach, circulating levels of K1 may be increased by at least 3-fold if the greens are accompanied by a little vegetable oil (unhydrogenated is preferred for the reasons discussed earlier); however, despite the fact that simultaneous intake of a small amount of fat improves K1 absorption significantly, absorption of MKs, which are derived mainly from fats and dairy products (including low-fat dairy products, which still contain some fat), is much better. 15
Vitamin K1’s Contributions to Healthy Aging
K1 Promotes Bone Quality not Quantity
It is becoming widely accepted that, in addition to BMD, healthy bone includes other qualitative properties, such as elasticity, structure and micro-architecture, which are now considered to be independent risk factors for bone fracture. Qualitative Ultrasound Assessment (QUS), in which these properties are evaluated, has therefore been suggested to provide better information than just BMD for estimating bone strength, and some consider QUS a promising alternative to DXA since it shows a significant correlation with DXA values in the lumbar spine. Several recent studies utilizing QUS suggest that K1 promotes better bone quality. 73, 74, 75, 76, 77
Both the Nurses’ Health Study and the Framingham Heart Study found a significantly lower relative risk of hip fracture in subjects with higher intake of K1. K1 intake of >242 μg/d was reported in the Nurses’ Health Study to lower RR of hip fracture by 30%, and in the Framingham Heart Study, the mean vitamin K intake, 254 μg/d, lowered RR by 65%. 78, 79, 80
Results from the ECKO trial also provide evidence of K1’s beneficial effect on bone quality. This was a 2-year double-blind randomized, placebo-controlled trial involving 440 postmenopausal women with osteopenia. K1 (at a pharmacological dose of 5 mg/day) did not provide any protection against age-related decline in BMD or bone resorption markers—even if the women’s vitamin D levels were adequate; however, K1 was found to provide significant protection against clinical fractures: only 9 women given vitamin K1 vs. 20 given placebo experienced fractures (and fracture prevention is the reason we treat!), suggesting that K1’s effect on bone health is not due to its effects on BMD or bone turnover. 11
Lead author, Cheung said she could not account for these highly beneficial outcomes. In an LMR review of this study, Vitamin K2, not KI, Helpful for Bone Density, we proposed that research indicating that vitamin K1 significantly lowers cytokine production and overall inflammation may provide one reason. It is well known that inflammation increases as estrogen levels decline with menopause, and that increased production of pro-inflammatory cytokines is associated with increased differentiation and activation of osteoclasts. Vitamin K lessens oxidative stress and down-regulates expression of pro-inflammatory cytokines. K2 also provides anti-inflammatory effects via carboxylation of Gas6 (growth arrest specific gene 6), which then speeds the phagocytosis of apoptotic cells (an essential process for normal tissue development and maintenance), and promotes cell survival in a wide range of cell types.
A more recent study, (Bullo et al., 2011), provides insight into how vitamin K1 supports elders’ bone health. This 2 year study followed a cohort of 200 people, average age 67 years, who had good nutritional status and consumed a traditional healthy Mediterranean diet rich in vitamin K. Analysis of bone health using QUS assessment revealed significant correlations between higher K1 intake and superior bone quality properties, lower losses of bone mineral density, and smaller increases in the porosity and elasticity attributed to aging. The authors note: “Since the participants in this analysis already ate a healthy diet rich in K1, even more beneficial effects may be seen in populations with a lower intake of vitamin K or poor nutrition. 78
K1’s Anti-Inflammatory Actions Protective vs. Osteoarthritis
An analysis of data collected on 672 participants in the Framingham Offspring cohort found a strong inverse association between circulating K1 and the prevalence of OA, occurrence of osteophytes (bone spurs) and joint space narrowing, indicating an association between low plasma levels of K1 and increased prevalence of OA in the hand and knee. 81
Vitamin K Promotes Detoxification
Vitamin K upregulates production of CYP3A isoforms by binding to and activating the steroid and xenobiotic receptor (SXR), a nuclear receptor that acts as a sensor for the presence of many drugs (e.g., phenobarbital, taxol, rifampicin) and initiates the transcription of CYP450 enzymes and transporter molecules that clear these substances from the body. MK-4, which has an unsaturated side chain, activates nuclear receptor SXR (which then induces CYP450 enzymes),8 -10 fold, while K1, which has a saturated side chain, causes a lesser 2-5 fold activation. 82
Vitamin K2’s binding to the SXR also induces bone-specific genes that favor the expression of osteoblastic markers – yet another way in which K2 (as MK-4 in this in vitro study) promotes bone formation. 83
Conclusion
Supplementation with Physiological Doses of Vitamin K — K1 plus MK-7 — Rather than Pharmacological Doses of MK-4, May Be Preferable
K1 can be consumed in sufficient amounts to carboxylate hepatic VKD proteins by eating leafy greens, whose increased consumption should be encouraged because they are also low in calories, high in fiber, and rich in numerous other micronutrients essential for healthy aging. However, while natto-eating Japanese can easily ingest sufficient amounts of natto to provide >150 mcg of MK-7 on a daily basis (1.5 ounces of natto contains ~ 435 mcg of MK-7), 84 the Western diet not only affords much lesser amounts of the MKs necessary for the carboxylation of the extra-hepatic VKD proteins, but its K2-rich foods are concentrated sources of calories and (with the exception of low-fat dairy products) saturated fat. Thus, the Western diet cannot provide adequate amounts of K2 to fully carboxylate extra-hepatic VKD proteins without increasing other risks for chronic degenerative disease. Ensuring protective levels of Vitamin K2 via a supplement along with low-fat dairy foods (for those not lactose-intolerant, nor reactive to dairy protein), is therefore advisable. The question then becomes, which supplemental form(s) of MK at what dosage would be best? We suggest somewhere in the range of 45 – 90 mcg K1 plus 100 – 200 mcg K2 as MK-7/day. The lower end of the dosage range is likely to be sufficient for apparently healthy individuals, particularly since MK-7 accumulates in tissues to provide a reserve, 9 while those with conditions related to vitamin K insufficiency may require the higher dose to help promote reversal of pathology.
In contrast to K1 and MK-4, which are cleared from the body within a matter of hours, the longer-chain MKs’ incorporation into LDL gives these forms of K2 several day half-lives during which they are transported from the liver to other tissues where they can be used to build and maintain a reserve that promotes optimal activation of the extra-hepatic VKD proteins. Expressed as AUC over 24 hours, the availability of MK-7 is 2.5-fold better than that of K1; expressed as AUC over 96 hours, it is 6-fold better. This greatly extended availability is what enables the long-chain MKs to optimize activation of extra-hepatic VKD proteins at a dose that could feasibly be supplied by the diet (150 mcg/day of MK-7) rather the pharmaceutical dose (15 mg t.i.d, i.e., 45 mg/day) required for MK-4. 58, 9
In experiments monitoring the efficacy of K1 and MK-7 for osteocalcin carboxylation (using the ratio between circulating cOC and ucOC), both vitamins induced increased OC carboxylation within 3 days, but only with MK-7 did ratio between cOC and ucOC continue to increase during the entire study periods, of 40 and 84 days, respectively. 9, 13 And the difference in carboxylation efficacy was quite significant: in both trials, MK-7 increased carboxylation 5-fold more than K1.
Present recommendations for vitamin K intake range from 90 to 120 mcg/day. In a dose of 45 mg/day, which must be given as 15 mg 3 times per day due to MK-4’s 6-8 hour biological half-life, the vitamin is being used as a drug not a nutritional supplement. In addition to the problems with patient compliance sure to occur with this 15 mg/t.i.d. regimen, MK-4’s short half-life will necessarily result in fluctuating K2 serum levels. At this time no data are available on the efficacy of MK-4 at lower doses.
In many species including humans, K1 is a minor constituent of hepatic vitamin K content with the majority being long-chain menaquinones, MK-7 through to MK-13. 5 Typically the ratio is about 90% menaquinones:10% phylloquinone. It is this author’s belief that the human body does not make mistakes. The fact that the body so quickly clears K1 and MK-4, while allowing the longer-chain MKs to accumulate, raises the question of “Why?” Why are these different forms of vitamin K treated so differently? Might there be untoward repercussions from ingestion of pharmacological levels of K1 or MK-4 over many years? The fact that vitamin K, at levels potentially consumed in a healthful diet, activates PXR, which then induces production of CYP450s responsible for the elimination of xenobiotics, suggests that pharmacological amounts of these vitamins might be considered by the body as potentially harmful xenobiotics. 82 Why take this risk when much smaller doses of MK-7, which could conceivably be ingested via consumption of a healthful diet, have been shown to accumulate in the body and effectively carboxylate extra-hepatic VKD proteins?
One Caveat for Patients on Coumarins (Oral Anticoagulants)
Supplemental (and dietary) vitamin K can interfere with the anticoagulant action of coumarin derivatives, but lower doses appear to be safe. Experiments have shown that only at a dose of 315 mcg/d did K1 affect INR, decreasing it from 2.0 to 1.5. As one might expect, MK-7 turned out to be much more potent; a comparable decrease in INR was reached at an intake of 130 mcg/d. For this reason, Schurgers et al. recommend an upper safety limit of 50 mcg/d for supplemental long-chain menaquinones (i.e., MK-7) in patients on oral anticoagulant treatment. This dose is comparable to the menaquinone content of 75 to 100 g of cheese, and research evaluating the effect on INR of gradually increasing doses of MK-7 indicate 50 mcg/day would lead to a disturbance of the INR value of no more than 10%. The 96 hour half-life of MK-7, however, suggests that regular intake of MK-7 in combination with properly adapted coumarin doses should result in more stable INR values. 9 Thus carefully monitored patients on courmarin derivatives may be able to take a higher daily dose of MK-7, a preferable prescription given the greatly increased risk of vascular calcification with coumarin use.
References
- Allison AC. The possible role of vitamin K deficiency in the pathogenesis of Alzheimer’s disease and in augmenting brain damage associated with cardiovascular disease. . Med Hypotheses. . 2001 Aug;57(2):151-5.. ↑
Abstract - K3 (menadione), a third, much simpler form of the vitamin, is considered a synthetic analogue, although intestinal bacteria can produce extremely minute amounts from K1. K3 has been utilized in . cancer research because it potentiates the cytotoxic activity of chemotherapeutic agents and vitamin C.. “Vitamin D and Vitamin K Team Up to Lower CVD Risk, Part II: The Vitamin K Connection to Cardiovascular Health”. ↑
- Graphic may be accessed @ Wikipedia: . ↑
http://en.wikipedia.org/wiki/Vitamin_K - Vitamin K, Food Sources, Linus Pauling Institute, accessed 7-12-11: . ↑
http://lpi.oregonstate.edu/infocenter/vitamins/vitaminK/index.html#food_source - Shearer MJ, Newman P.. Metabolism and cell biology of vitamin K. . Thromb Haemost.. 2008 Oct;100(4):530-47. . ↑
Abstract - Schurgers LJ, Vermeer C. . Determination of phylloquinone and menaquinones in food: effect of food matrix on circulating vitamin K concentrations.. Haemostasis . 2000;30:298–307. . ↑
Abstract - Vitamin K. www.WHFoods.org. accessed 7-12-11 . ↑
http://www.whfoods.org/genpage.php?tname=nutrient&dbid=112#foodsources - Schurgers LJ, Geleijnse JM, Grobbee DE, et al. . Nutritional intake of vitamins K1 (phylloquinone) and K2 (menaquinone) in the Netherlands.. J,Nutr. Environ. Med. . June 1999;9(2):115–122. DOI: 10.1080/13590849961717 accessed at Ingentaconnect 7-15-11 @ . ↑
http://www.ingentaconnect.com/content/routledg/cjne/1999/00000009/00000002/art00004 - Schurgers LJ, Teunissen KJ, Hamulyák K, et al. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. . Blood. . 2007 Apr 15;109(8):3279-83. Epub 2006 Dec 7. . ↑
Abstract - Yamauchi M, Yamaguchi T, Nawata K, et al.. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women.. Clin Nutr. . 2010 Dec;29(6):761-5. Epub 2010 Mar 23.. ↑
Abstract - Cheung AM, Tile L, Lee Y, et al. . Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. . PLoS Med. . 2008 Oct 14;5(10):e196.. ↑
Abstract - Yasui T, Miyatani Y, Tomita J, et al. . Effect of vitamin K2 treatment on carboxylation of osteocalcin in early postmenopausal women. . Gynecol Endocrinol. . 2006 Aug;22(8):455-9. . ↑
Abstract - Vermeer C, Edwall D. . Health effects of vitamin K2. , presentation at The Bone & Joint Health . 2011 virtual conference 5-25-11. ↑
- Schurgers LJ, Teunissen KJ, Knapen MH, et al. . Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. . Arterioscler Thromb Vasc Biol. . 2005 Aug;25(8):1629-33.. ↑
Abstract - Geleijnse JM, Vermeer C, Grobbee DE, et al.. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. . J Nutr. 2004 Nov;134(11):3100-5. . ↑
Abstract - Schurgers LJ, Cranenburg EC, Vermeer C.. . Matrix Gla-protein: the calcification inhibitor in need of vitamin K. . Thromb Haemost. . 2008 Oct;100(4):593-603. . ↑
Abstract - Pizzorno L.. Vitamin D and Vitamin K Team Up to Lower CVD Risk: Part II, The Vitamin K Connection to Cardiovascular Health. Longevity Medicine Review, access @ . May 2009. ↑
http://www.lmreview.com/articles/view/vitamin-d-and-vitamin-k-team-up-to-lower-cvd-risk-part-II/ - Viegas CS, Cavaco S, Neves PL, et al. . Gla-rich protein is a novel vitamin K-dependent protein present in serum that accumulates at sites of pathological calcifications.. . Am J Pathol. . 2009 Dec;175(6):2288-98. . ↑
Abstract - Schurgers LJ, Barreto DV, Barreto FC, et al. . The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010 Apr;5(4):568-75. Epub 2010 Feb 4. . ↑
Abstract - Mizobuchi M, Towler D, Slatopolsky E. . Vascular calcification: the killer of patients with chronic kidney disease. . J Am Soc Nephrol. 2009 Jul;20(7):1453-64. . ↑
Abstract - Schlieper G, Westenfeld R, Krüger T, et al. . Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD.. J Am Soc Nephrol. 2011 Feb;22(2):387-95. . ↑
Abstract - Fusaro M, Crepaldi G, Maggi S, et al. . Vitamin K, bone fractures, and vascular calcifications in chronic kidney disease: an important but poorly studied relationship.. J Endocrinol Invest. 2011 Apr;34(4):317-23. . ↑
Abstract - Cranenburg EC, Koos R, Schurgers LJ, et al. . Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. . 2010 Oct;104(4):811-22. Epub 2010 Aug 5. . ↑
Abstract - Bolland MJ, Grey A, Avenell A, et al. . Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ.. 2011 Apr 19;342:d2040. ↑
Abstract - Wallace RB, Wactawski-Wende J, O’Sullivan MJ, et al.. Urinary tract stone occurrence in the Women’s Health Initiative randomized clinical trial of calcium and vitamin D supplements. Am J Clin Nutr. 2011 Apr 27. [Epub ahead of print] . ↑
Abstract - Masterjohn C. . Vitamin D toxicity redefined: vitamin K and the molecular mechanism. . Med Hypotheses.. 2007;68(5):1026-34. . ↑
Abstract - Adams J, Pepping J. . Vitamin K in the treatment and prevention of osteoporosis and arterial calcification. Am J Health Syst Pharm.. . 2005 Aug 1;62(15):1574-81. . ↑
Abstract - Price PA, Faus SA, Williamson MK. . Warfarin-induced artery calcification is accelerated by growth and vitamin D.. Arterioscler Thromb Vasc Biol. 2000 Feb;20(2):317-27. . ↑
Abstract - Spronk HM, Soute BA, Schurgers LJ, et al.. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats.. J Vasc Res. . 2003 Nov-Dec;40(6):531-7. . ↑
Abstract - Sato Y, Honda Y, Hayashida N, et al. . Vitamin K deficiency and osteopenia in elderly women with Alzheimer’s disease. Arch Phys Med Rehabil. 2005 Mar;86(3):576-81. . ↑
Abstract - Presse N, Shatenstein B, Kergoat MJ, et al. . Low vitamin K intakes in community-dwelling elders at an early stage of Alzheimer’s disease.. J Am Diet Assoc. . 2008 Dec;108(12):2095-9. . ↑
Abstract - Rosand J, Hylek EM, O’Donnell HC, et al. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000 Oct 10;55(7):947-51. ↑
Abstract - Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005 Aug;3(8):1873-8. Review. ↑
Abstract - Cauley JA, Zmuda JM, Yaffe K, et al. . Apolipoprotein E polymorphism: A new genetic marker of hip fracture risk–The Study of Osteoporotic Fractures.. . J Bone Miner Res. 1999 Jul;14(7):1175-81. ↑
Abstract - Johnston JM, Cauley JA, Ganguli M.. APOE 4 and hip fracture risk in a community-based study of older adults. . J Am Geriatr Soc. . 1999 Nov;47(11):1342-5. . ↑
Abstract - Pauli RM, Lian JB, Mosher DF, et al. . Association of congenital deficiency of multiple vitamin K-dependent coagulation factors and the phenotype of the warfarin embryopathy: clues to the mechanism of teratogenicity of coumarin derivatives. Am J Hum Genet. 1987 Oct;41(4):566-83. . ↑
Abstract - Koebert MK, Haun JM, Pauli RM. . Temporal evolution of risk estimates for presumed human teratogens.. Reprod Toxicol.. . 1993 Jul-Aug;7(4):343-8. . ↑
Abstract - Crivello NA, Casseus SL, Peterson JW, et al. . Age- and brain region-specific effects of dietary vitamin K on myelin sulfatides. . J Nutr Biochem. 2010 Nov;21(11):1083-8. . ↑
Abstract - Troy LM, Jacques PF, Hannan MT, et al. . Dihydrophylloquinone intake is associated with low bone mineral density in men and women. Am J Clin Nutr. . 2007 Aug;86(2):504-8. . ↑
Abstract - Booth SL, Lichtenstein AH, O’Brien-Morse M, et al. . Effects of a hydrogenated form of vitamin K on bone formation and resorption.. Am J Clin Nutr. 2001 Dec;74(6):783-90. . ↑
Abstract - Yoshida M, Booth SL, Meigs JB, et al. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am J Clin Nutr. . 2008 Jul;88(1):210-5. . ↑
Abstract - Yoshida M, Jacques PF, Meigs JB, et al. . Effect of vitamin K supplementation on insulin resistance in older men and women. . Diabetes Care.. 2008 Nov;31(11):2092-6. Epub 2008 Aug 12. ↑
Abstract - Beulens JW, van der ADL, Grobbee DE, et al.. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. .. Diabetes Care.. 2010 Aug;33(8):1699-705. Epub 2010 Apr 27. ↑
Abstract - Gundry SR. . Dr. Gundry’s Diet Evolution: Turn Off the Genes That Are Killing You and Your Waistline. . Three Rivers Press: . March, 2009.. ↑
- Pizzorno L. . Vitamin K: beyond coagulation to uses in bone, vascular and anti-cancer metabolism. IMCJ 2008 . Apr; 7(2): 24-30, IMCJ subscriber access at . ↑
http://www.imjournal.com/index.cfm/fuseaction/archives.display/action/download/id/8350 - Motyl KJ, McCabe LR, Schwartz AV. Bone and glucose metabolism: a two-way street. Arch Biochem Biophys. 2010 Nov 1;503(1):2-10. Epub 2010 Aug 1. . ↑
Abstract - Lee NK, Sowa H, Hinoi E, et al. . Endocrine regulation of energy metabolism by the skeleton. Cell.. 2007 Aug 10;130(3):456-69. . ↑
Abstract - Kaneki M. Is ucOC a novel bone-derived anti-diabetogenic hormone in humans?. Clin Calcium. . 2009 Sep;19(9):1304-10. . ↑
Abstract - Kanazawa I, Yamaguchi T, Yamamoto M, et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus.. J Clin Endocrinol Metab. . 2009 Jan;94(1):45-9. Epub 2008 Nov 4. . ↑
Abstract - Shea MK, Gundberg CM, Meigs JB, et al.. Gamma-carboxylation of osteocalcin and insulin resistance in older men and women. . Am J Clin Nutr. 2009 Nov;90(5):1230-5. Epub 2009 Sep 23. . ↑
Abstract - Im JA, Yu BP, Jeon JY, et al. . Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta. 2008 Oct;396(1-2):66-9. . ↑
Abstract - Suh SH. Integrative physiology: defined novel metabolic roles of osteocalcin. J Korean Med Sci. . 2010 Jul;25(7):985-91. . ↑
Abstract - Kanazawa I, Yamaguchi T, Yamamoto M, et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009 Jan;94(1):45-9. . ↑
Abstract - Razzaque MS. . Osteocalcin: a pivotal mediator or an innocent bystander in energy metabolism?. Nephrol Dial Transplant.. 2011 Jan;26(1):42-5. Epub 2010 Dec 3. . ↑
Abstract - Foresta C, Strapazzon G, De Toni L et al. . Evidence for osteocalcin production by adipose tissue and its role in human metabolism.. . J Clin Endocrinol Metab. 2010 Jul;95(7):3502-6. Epub 2010 Apr 21. . ↑
Abstract - Pizzorno LE, Wright JV. Your Bones. Praktikos Books: Mt. Jackson, VA,. 2011, p. 154, 213.. ↑
- Tobin BW, Uchakin PN, Leeper-Woodford SK. Insulin secretion and sensitivity in space flight: diabetogenic effects. Nutrition. 2002 Oct;18(10):842-8.. ↑
Abstract - Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta . 2002 Feb 15;1570(1):27-32. . ↑
Abstract - Nimptsch K, Rohrmann S, Linseisen J. . Dietary intake of vitamin K and risk of prostate cancer in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg) . Am J Clin Nutr. 2008 Apr;87(4):985-92. . ↑
Abstract - Nimptsch K, Rohrmann S, Kaaks R, et al. . vitamin K intake in relation to cancer incidence and mortality: results from the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg).. Am J Clin Nutr. 2010 May;91(5):1348-58. ↑
Abstract - McCann JC, Ames BN. . Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging?. Am J Clin Nutr. 2009 Oct;90(4):889-907. Epub 2009 Aug 19. . ↑
Abstract - Mizuta T, Ozaki I. . Hepatocellular carcinoma and vitamin K. Vitam Horm.. 2008;78:435-42. . ↑
Abstract - Nakagawa K, Hirota Y, Sawada N, et al.. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme.. Nature.. 2010 Nov 4;468(7320):117-21. . ↑
Abstract - Nimptsch K, Rohrmann S, Nieters A, et al.. Serum undercarboxylated osteocalcin as biomarker of vitamin K intake and risk of prostate cancer: a nested case-control study in the Heidelberg cohort of the European prospective investigation into ca. Cancer Epidemiol Biomarkers Prev. . 2009 Jan;18(1):49-56. ↑
Abstract - Vermeer C, Theuwissen E. . Vitamin K, osteoporosis and degenerative diseases of ageing.. Menopause Int. . 2011 Mar;17(1):19-23. ↑
Abstract - Gast GCM, de Roos NM, Sluijs I, et al. . A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. 2009 Sep;19(7):504-10. . ↑
Abstract - Beulens JWJ, Bots ML, Atsma F, et al. . High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009 Apr;203(2):489-93. ↑
Abstract - Erkkila AT, Booth SL, Hu FB, et al. . Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women.. Eur J Clin Nutr. 2005 Feb;59(2):196-204. . ↑
Abstract - Erkkila AT, Booth SL, Hu FB, et al. . Phylloquinone intake and risk of cardiovascular diseases in men. Nutr Metab Cardiovasc Dis.. 2007 Jan;17(1):58-62. ↑
Abstract - Tanko LB, Christiansen C, Cox DA, et al. . Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res . 2005;20:1912e20. . ↑
Abstract - Gerber Y, Melton LJ 3rd, Weston SA, et al.. Between Myocardial Infarction and Fractures: An Emerging Phenomenon. . Circulation.. 2011 Jun 27. [Epub ahead of print] . ↑
Abstract - Vermeer C, Braam L. . Role of K vitamins in the regulation of tissue calcification. J Bone Miner Metab. 2001;19(4):201-6. . ↑
Abstract - Marin F, Lopez-Bastida J, Diez-Perez A, et al. . Bone mineral density referral for dual-energy X-ray absorptiometry using quantitative ultrasound as a prescreening tool in postmenopausal women from the general population: a cost-effectiveness analys. Calcif Tissue Int. 2004 Mar;74(3):277-83. ↑
Abstract - Krieg MA, Barkmann R, Gonnelli S, et al.. Quantitative ultrasound in the management of osteoporosis: the 2007 ISCD official positions.. J Clin Densitom. 2008 Jan-Mar;11(1):163-87.. ↑
Abstract - Moris M, Peretz A, Tjeka R, et al. . Quantitative ultrasound bone measurements: normal values and comparison with bone mineral density by dual X-ray absorptiometry. Calcif Tissue Int. . 1995 Jul;57(1):6-10. . ↑
Abstract - Laugier P. . Quantitative ultrasound of bone: looking ahead. .. Joint Bone Spine. 2006 Mar;73(2):125-8. . ↑
Abstract - Lappa V, Dontas IA, Trovas G, et al. . Quantitative ultrasound is better correlated with bone mineral density and biochemical bone markers in elderly women.. Clin Rheumatol. 2007 Jul;26(7):1067-73. ↑
Abstract - Bulló M, Estruch R, Salas-Salvadó J.. Dietary vitamin K intake is associated with bone quantitative ultrasound measurements but not with bone peripheral biochemical markers in elderly men and women. Bone. . 2011 Jun 1;48(6):1313-8. . ↑
Abstract - Feskanich D, Weber P, Willett WC, et al. . Vitamin K intake and hip fractures in women: a prospective study. . Am J Clin Nutr. 1999 Jan;69(1):74-9. . ↑
Abstract - Booth SL, Tucker KL, Chen H, et al. . Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. . Am J Clin Nutr. 2000 May;71(5):1201-8. . ↑
Abstract - Neogi T, Booth SL, Zhang YQ, et al. . Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum. . 2006 Apr;54(4):1255-61.. ↑
Abstract - Landes N, Birringer M, Brigelius-Flohé R.. Homologous metabolic and gene activating routes for vitamins E and K. Mol Aspects Med. 2003 Dec;24(6):337-44. . ↑
Abstract - Tabb MM, Sun A, Zhou C, et al. . Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. . J Biol Chem. 2003 Nov 7;278(45):43919-27. ↑
Abstract - Yamaguchi M. . Regulatory mechanism of food factors in bone metabolism and prevention of osteoporosis. . Yakugaku Zasshi. 2006 Nov;126(11):1117-37. . ↑
Abstract

Comments are closed.