Abstract
In just the last 30 years, molecular biologists have discovered genes with the potential to extend longevity: SIR2 in yeast and its mammalian equivalent, SIRT1. In animal models, SIRT1, via the enzymes it encodes, has been shown to have healthspan extending regulatory effects on metabolism, stress resistance, cellular survival, cellular senescence, inflammation-immune function, endothelial function, and circadian rhythms. Clinically relevant findings first began to appear in the peer-reviewed medical literature a mere 10 years ago with the revelation that the keys that unlock and activate our longevity genes are naturally occurring dietary polyphenols.
Part I of this review highlights key historical high points of the research odyssey that cracked the longevity code and provides the reader with a backstage pass to meet the enzymes encoded by SIRT1—the seven nutrient-responsive NAD+-dependent histone deacetylase enzymes, dubbed the sirtuins. Part I’s interview with these intriguing enzymes provides answers to the following questions: What are the unique nutrient requirements of the sirtuins? How and why is their activation impacted not only by calorie restriction, but by certain phytonutrients such as resveratrol, and by the redox state of the cell, specifically its NAD+/NAD(H) ratio? What can be done to optimize this ratio via NAD+ salvage, and why will this—in conjunction with resveratrol and/or other SIRT1-activating compounds–optimize sirtuin activity? Why might a very recently discovered niacin-related compound, nicotinamide riboside, be a better sirtuin-activating partner for resveratrol than niacin (nicotinic acid) or its derivative, niacinamide?
Part II of this review details SIRT1’s anti-aging effects via an overview of the primary functions of key regulatory proteins deacetylated by Sirt1 and the mitochondrial sirtuins (sirtuins 3-5). Even the brief overview provided demonstrates why the sirtuins, nicknamed “the magnificent seven” have also been dubbed “the master switches of metabolism.” Although resveratrol’s beneficial effects are primarily mediated through its activation of SIRT1, resveratrol’s cellular targets also include a number of other important regulatory enzymes including cyclooxygenases, lipooxygenases, kinases, ribonucleotide reductase, adenylyl cyclase, aromatase and DNA polymerases. The potential impact on healthspan of resveratrol’s modulation of the activity of these extra-sirtuin targets is outlined. Lastly, a number of other naturally occurring dietary polyphenols have lately been recognized to be sirtuin activating compounds (STACs); these, and their common mechanism of action, are noted.
We have entered a new era in which the key genes and intracellular pathways responsible for aging and longevity have been identified. Our discovery of the sirtuins – and the fact that their activity is responsive to changes in cellular metabolism and a number of xenohormetic plant compounds –has proven that lifespan is tractable. Not only is the research progressing at an exponential rate, but, right now—today, we can safely utilize emerging knowledge to develop effective longevity-enhancing protocols.
SIRT1 Longevity-Extending Mechanisms of Action
As noted in Part I of this review, Sirt1, the most researched of the sirtuin enzymes, is known to deacetylate both histones (e.g., H1, H3), and a number of non-histone regulatory protein substrates including LXR, FOXO, PGC-1α, NFκB, Ku70, mTOR and p53 (discussed below). Other sirtuins add acetyl Co-A, Complex I, glutamate dehydrogenase, insulin degrading enzyme, carbamoyl phosphate synthetase and DNA polymerase 1 to the long list of key regulatory proteins modulated by sirtuin activity. With an enzymatic finger in so many metabolic pies, the sirtuins have been dubbed the “master switches of metabolism.”1
This review will only attempt to list the primary functions of key histone and non-histone proteins deacetylated by Sirt1 and those of the key enzymes regulated by sirtuins 3-5 (a.k.a., the mitochondrial sirtuins).
SIRT1 regulation of age-related physiology
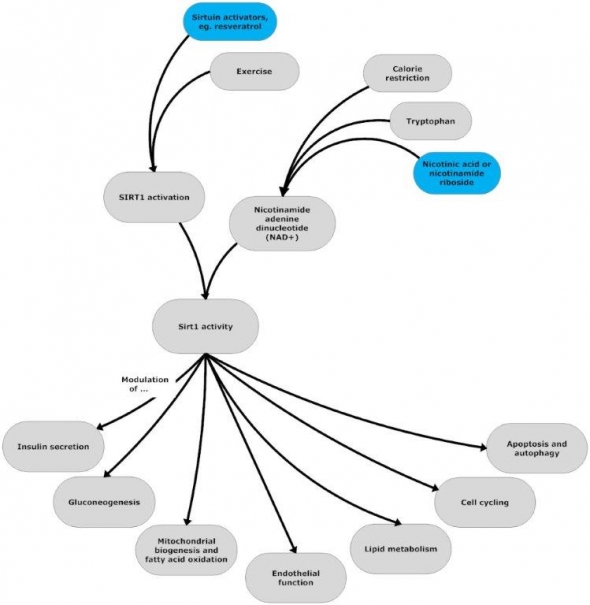
Flowchart by John Morgenthaler
Calorie restriction / Sirtuin activators, eg. resveratrol / Exercise2 —all increase sirtuin enzyme expression and activity, thus modulating numerous longevity-promoting cellular functions. SIRT1 and its enzymes act as a cellular redox /energy sensor, responding to the current state of the cell by deacetylating a wide array of pivotal metabolic transcription factors that modulate insulin secretion, gluconeogenesis, mitochondrial biogenesis and fatty acid oxidation, endothelial function, lipid metabolism, cell cycling, apoptosis and autophagy.
Key Sirt1 Targets: Primary Functions and Clinical Relevance
Histone 1: Genomic Stability, Cellular Longevity & Cancer Prevention
As noted above, the building block of chromatin is the nucleosomal core particle. Each of these contains an histone octamer around which 147 base pairs of DNA are wrapped. H1 binds to the DNA located at the entry and exit of these nucleosomal core particles, linking them together.3 H1 therefore has major impact on not only the formation and stability of chromatin, but also modulates chromatin remodeling, an integral part of the DNA repair response to damage.45
Histone 3: Telomere Protection, Preventing Premature Cellular Senescence
An histone found in telomeric chromatin that, when acetylated by Sirt1, is involved in “telomeric silencing,” the way in which telomeres protect chromosome ends from the potentially overly efficient systems that monitor and repair DNA double-strand breaks (DSBs). The idea here is to prevent these systems from causing the fusion of telomeres either to themselves or to accidentally generated DSBs as either would have dire consequences for the cell.6
Ku70: More Protection for Telomeres
A non-histone protein, Ku70, together with Ku80, makes up the Ku heterodimer, which is involved in maintaining telomere length. When deacetylated, Ku70 helps protect telomere ends from fusion events. Sirt1’s effect on Ku70 is another of the ways in which Sirt1 helps maintain telomere structure and prevents the telomere from being inappropriately fused.6 During conditions of oxidative stress, Ku70 is acetylated, which lessens its ability to bind a pro-apoptotic protein called Bax. By deacetylating Ku70, Sirt1 causes Bax to remain bound to Ku70, preventing Bax from initiating apoptosis. (Sirt1 also acts as a tumor suppressor by initiating the BAX pathway when appropriate—see below: Sirt1 prevents p53’s anti-cancer action at the nucleus, but promotes p53’s apoptotic effect via the BAX pathway.)7
PGC-1α: Improved Glucose Sensitivity & Mitochondrial Function = Weight Loss, Increased Endurance
Peroxisome proliferator-activated receptor gamma coactivator-1-alpha (PGC-1α) induces expression of genes in skeletal muscle for gluconeogenesis, mitochondrial fatty acid oxidation, and mitochondrial biogenesis. Treatment with resveratrol that results in Sirt1 deacetylation of PGC-1α rapidly improves insulin sensitivity and glucose tolerance in obese mice; a longer treatment course lowers body weight, induces mitochondrial biogenesis, eliminates ectopic fat deposits, and increases endurance.891011
PPARγ: Decreased Lipogenesis Plus Increased Lipolysis of Stored Fat = Weight Loss
Peroxisome proliferator-activated receptor gamma SIRT1 (PPARγ) deacetylation by Sirt1 results in repression of transcription of its target genes that are involved in fat storage. Upregulation of SIRT1 in differentiated fat cells triggers lipolysis and results in decreased fat storage in white adipose tissue.
PPARα: Increased Use of Fatty Acids in Mitochondrial Energy Production = Weight Loss
Peroxisome proliferator-activated receptor alpha (PPARα) induces genes involved in fatty acid uptake and oxidation, and suppresses glycolysis in the liver. Key PPARα target genes include carnitine palmitoyltransferase I (CPT I), involved in the transport of long-chain fatty acyl goups into the mitochondria; medium-chain acyl-CoA dehydrogenase (involved in β-oxidation in the mitochondria) and, specifically in liver, mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase (the rate limiting enzyme of ketogenesis). Sirt1 induces PGC-1α, which then induces transcription of PPARα gene targets.8912
UCP2: Improved Pancreatic Function / Insulin Secretion
Uncoupling protein gene 2 is repressed by Sirt1, which binds directly to the UCP2 promoter. Suppression of UCP2 restores beta cells’ ability to secrete insulin and pancreatic beta cell survival.13
eNOS: Improved Endothelial, Cardiovascular and Erectile Function
Endothelial nitric oxide synthase, an enzyme that generates nitric oxide, is atheroprotective, helps suppress inflammation and the production of reactive oxygen and reactive nitrogen species in arteries, and promotes blood vessel relaxation. (For LMR reviews discussing eNOS’ critical importance to cardiovascular and sexual health, please see Parts I and II of Longevity Medicine Strategies for CVD: Closing the Statin Gap, and Managing Erectile Dysfunction—When Viagra Doesn’t.) SIRT1 deacetylates lysines 496 and 506 in the calmodulin-binding domain of eNOS, which leads to enhanced eNOS activity.9
LXR: Improved HDL Cholesterol Production / LDL Cholesterol Efflux
The Liver X receptor is a member of the nuclear receptor family of transcription factors and closely related to nuclear receptors such as PPAR, FXR and RXR. LXR proteins function as cholesterol sensors and regulate whole-body cholesterol and lipid homeostasis. By deacetylating the LXR, Sirt1 causes transcription of numerous proteins involved in lipid metabolism, including the ABCA1 transporter (a.k.a. cholesterol efflux regulatory protein [CERP]), a major regulator of cellular cholesterol and phospholipid homeostasis, which mediates an early step in HDL biogenesis), thus promoting cholesterol efflux. 14
SREBP1: Inhibition of Triglyceride Synthesis, Protection against Non-Alcoholic Fatty Liver Disease
Sterol regulatory element binding protein1 (SREBP1) expression and activity is downregulated in hepatic cells by resveratrol via its upregulation of the expression of Sirt1 and FOXO1. SREBP1 controls hepatic triglyceride synthesis, thus its inhibition not only lowers triglyceride levels systemically but lessens fat deposition in the liver, which may be useful in the treatment of NAFLD (non-alcoholic fatty liver disease).15
Atg5, Atg7, Atg8: Promoting Cellular Rejeuvenation via Activation of Key Autophagy Genes
Sirt 1 forms a molecular complex with these genes that is required for autophagy. Accumulation of damaged proteins and organelles is an important aspect of the aging process that accelerates age-related pathologies. Basal levels of autophagy, the process through which damaged cellular components are broken down and recycled, are elevated during caloric restriction, primarily via augmented Sirt1 expression.16
mTOR and FOXO: Key Transcription Factors Required for Autophagy
mTOR is an evolutionarily-conserved protein kinase whose deacetylation by Sirt1 is required for full induction of autophagy.17
The FOXO (Forkhead Box class O) proteins are a family of transcription factors that regulate genes involved in cellular catabolism and renewal. Through complex modulation of at least three of the four mammalian FOXO transcription factors, Sirt1 stimulates autophagic degradation in a way that enhances cellular cleansing to ensure that waste products do not accumulate as the cell ages. Sirt1 stimulates FOXO transcription factors that increase autophagic degradation of targeted proteins, while also controlling resistance to oxidative stress and increasing adipocytes’ production of adiponectin; that latter results in increased lipid catabolism, gluconeogenesis, triglyceride clearance, insulin sensitivity, and inhibition of adipogenesis.181920 Sirt1’s interactions with the FOXO family also promote longevity via modulating immune function. (See below: FOXO: Modulating Immune Function to Support Stress Resistance and Neuroprotection) For a full discussion of the central importance of autophagy for longevity, please see our LMR review, Beyond the Mitochondrial Tune Up: Part III.
p53: Sirt1’s Global Actions Promote Cell Survival, but Not Cancer
p53 is a tumor suppressor gene, thus its deacetylation is a potentially oncogenic action for Sirt1 since reducing p53 transactivation allows cells to bypass p53-mediated apoptosis. However, Sirt1 also plays a critical role in DNA-strand break repair and prevents tumorigenesis in mouse models of cancer. In models of leukemia and colon cancer, mice with additional copies of SIRT1 live longer. It’s complicated, but now appears that Sirt1 acts within feedback loops that allow it to promote cell survival in critical cell types without causing cancer.921
Two examples (immediately following) of such feedback loop interactions are provided by Sirt1’s interactions with Rb/E2F1and c-Myc.
Rb/ E2F1: Inducing growth arrest in cancer cells
Sirt1 interactions with retinoblastoma tumor suppressor protein, Rb, help prevent oncogenesis. Rb plays a major role in regulating mammalian cell cycle progression, mainly by targeting the family of transcription factors encoded by the E2F genes and repressing their transcriptional activity. Multiple genes involved in DNA synthesis and cell cycle progression are regulated by E2F transcription factors, and Rb prevents their expression by inhibiting E2F gene activity, thus inducing growth arrest. Sirt1 is involved in Rb-mediated repression of E2F activity.22
In addition, a negative feedback loop exists between the cell-cycle and apoptosis regulator E2F1 and Sirt1 that contributes to protection against survival of DNA-damaged (potentially cancerous) cells. E2F1 induces Sirt1 expression at the transcriptional level. Sirt1 binds to E2F1 and inhibits E2F1 activities, forming a negative feedback loop.23
c-Myc: Another mechanism via which Sirt1 prevents rapid growth of cancer cells
c-Myc is a type of mRNA that serves as a template for the MYC protein, which is involved in the rapid growth of cancer cells. c-Myc binds to the SIRT1 promoter and induces SIRT1 activation. However, Sirt1 interacts with and deacetylates c-Myc, resulting in decreased c-Myc stability. As a consequence, Sirt1 undercuts c-Myc’s transformational capability. Experiments have identified a “c-Myc-Sirt1” feedback loop in the regulation of c-Myc activity and cellular transformation that supports a role for Sirt1 in tumor suppression.2425
Sirt1 prevents p53’s anti-cancer action at the nucleus, but promotes p53’s apoptotic effect via the BAX pathway
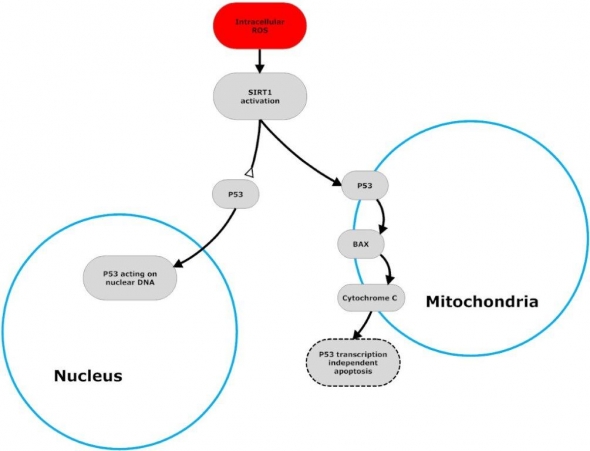
In response to increased intracellular oxidative stress, Sirt1 in the cytoplasm is activated, and binds to and deacetylates p53, blocking its nuclear translocation. Deacetylated p53 then translocates to the mitochondrial outer membrane and releases the pro-apoptotic protein BAX. Activated BAX causes the release of Cytochrome c from mitochondria to cytoplasm. Cytochrome c is a heme protein component normally located in the electron transport chain, but, when released into the cytoplasm, Cytochrome c initiates apoptosis by binding to apoptotic protease activating factor. Bottom line: under conditions of potentially oncogenic intracellular stress, Sirt1 utilizes p53 to initiate apoptosis.26
Sirt1 and p53 work together to promote genomic integrity
The most recent findings suggest that genomic integrity and stability require cooperation between p53 and Sirt1. Reduced levels of SIRT1 mRNA are seen in prostate and bladder carcinoma, glioblastoma, ovarian and mutated BRCA1 and 2-related breast cancer compared to normal tissues. (BRCA1 and 2 are tumor suppressor genes.) Sirt1 expression is higher in wild-type (protective) BRCA1. Mutated BRCA is associated with significantly increased breast cancer risk.9
p73: An oncoprotein suppressed by Sirt1
Further evidence that Sirt1 is not oncogenic is its deacetylation/suppression of p73, which is highly expressed in many tumors typically found in humans, including in breast and ovarian cancer, and is now thought to be an oncoprotein. Adenoviruses (so called because they were first isolated in the adenoids and are responsible for ~5-10% of respiratory infections) that cause cellular transformations also increase p73 expression. Deregulated over-expression of transcription factors involved in cell cycle progression (e.g., E2F-1, whose inhibition by Sirt1 is discussed above ), induces the expression of p73.2728 In sum, it’s a complicated web, but one in which SIRT1 and Sirt1 appear, overall, to not promote cancer. Still, this remains an area of controversy and on-going discovery.
Flowchart by John Morgenthaler
Flow chart adapted from Vijg J, Maslov A, Suh Y. Aging: a sirtuin shake-up? Cell. 2008 Nov 28;135(5):797-8.
NFκB: Preventing Excessive Inflammation
Nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), a master regulator of both adaptive and innate immunity, is a cytoplasmic sensor of danger signals including hypoxia, oxidative stress and genotoxic stress, whose activation results in the activation of pro-inflammatory genes. Sirt1 suppresses NF-kB binding to the nucleus (and therefore NFkB activation of pro-inflammatory genes) by deacetylating the RelA/p65 subunit, which is involved in its NFκB’s DNA binding. This results in significant lessening of inflammation, with widespread beneficial effects impacting conditions from autoimmunity to chronic obstructive pulmonary disease (COPD) to cardiovascular diseases, type 2 diabetes, Alzheimer’s and cancer. Macrophages and endothelial cells treated with Sirt1 activators (e.g., resveratrol) have much lower levels of inflammatory cytokines, including TNFα, intercellular adhesion molecule (ICAM)-1, interleukin (IL)-6, IL-1, and inducible NOS (iNOS).929
FOXO: Modulating Immune Function to Support Stress Resistance and Neuroprotection
Acetylation of FOXO is associated with inflammatory cell activation, endothelial angiogenic activity, and cellular apoptosis, including apoptosis of neuronal, vascular and cardiomyocyte cells. Sirt1 deacetylation of FOXO increases the number and function of regulatory T cells, which all by itself would modulate the immune response away from cell death and towards stress resistance. Current thinking, however, is that deacetylation of FOXO by Sirt1 leads to activation of a whole set of stress-resistance factors, tipping the balance toward stress resistance and away from apoptosis—a highly neuroprotective effect in diseases such as Alzheimer’s, Parkinson’s and Amyotrophic Lateral Sclerosis, and also protective in optic neuritis and Age-related Macular Degeneration. As noted in the above discussion of p53, an intricate interplay is constantly occurring among FOXOs, Sirt1, and p53, with each responding to the cellular context to regulate the other two.26
The Mitochondrial Sirtuins
While Sirt1 is the most studied, other sirtuins—also activated by resveratrol and other xenohoremetic compounds—have notable longevity-promoting effects. The research is just starting to develop on what have been called “the mitochondrial sirtuins,” sirtuins 3, 4 and 5. As our very brief summary of the research on these sirtuins below suggests, each could be the topic of its own LMR review.9
Sirtuin 3: A Mitochondrial Tumor Suppressor that Boosts ATP Production
Sirt3 binds to complex I in the electron transport chain (ETC), regulating its activity and energy levels in the cell. Sirt3 also binds and deacetylates acetyl-CoA synthetase 2 (which joins acetate and coenzyme A to produce acetyl-CoA for use in the Krebs cycle) and glutamate dehydrogenase (GDH, converts glutamate to α-Ketoglutarate, an intermediate in the Krebs cycle). Sirt3 deacetylation activates these enzymes. In cardiomyocytes, Sirt3 levels are elevated in both the mitochondria and nucleus.
Sirt3 also promotes cellular longevity by preventing apoptosis while acting as a tumor suppressor. Sirt3 deacetylates Ku70, preventing Bax activation, which triggers apoptosis. Most recently, Sirt3 has been shown to be a mitochondria-localized tumor suppressor that works its anti-oncogenic magic by enhancing expression of mitochondrial MnSOD (a key mitochondrial antioxidant that reduces superoxide, a highly promiscuous free radical produced as a byproduct in ETC reactions).303132 For a full discussion of mitochondrial energy production, please see our LMR review: Beyond the Mitochondrial Tune Up, Part I.
Sirtuin 4: Regulating Amino Acid Metabolism and Blood Glucose Levels
Sirt4 binds and represses GDH activity via ADP-ribosylation. Beta cells secrete insulin in response to an increase in the ADP:ATP ratio. As amino acids are broken down by GDH into α-ketoglutarate, this ratio rises, and more insulin is secreted. By repressing GDH activity, Sirt4 regulates the metabolism of amino acids, thus controlling insulin secretion and regulating blood glucose levels.933
Sirtuin 5: Ensuring Healthy Protein Metabolism
Sirt5 deacetylates and activates carbamoyl phosphate synthetase 1 (CPS1), the rate-limiting step of the urea cycle, a key component of protein metabolism and clearance of its waste product (ammonia).9
Resveratrol: Extra-Sirtuin Life-Extending Benefits
While it appears that the beneficial effects of resveratrol are primarily mediated through its activation of SIRT1, the sirtuins are not the only mediator of resveratrol’s complex longevity promoting effects—far from it! Resveratrol has numerous other cellular targets, some of whose activities it enhances while inhibiting others. These include cyclooxygenases, lipooxygenases, kinases, ribonucleotide reductase, adenylyl cyclase, aromatase and DNA polymerases.934
Here’s a very brief look at some of resveratrol’s extra-sirtuin targets:
Cyclo- and Lipooxygenases: Anti-inflammatory effects
The cyclooxygenases (COX-1 and COX-2)- and 5-lipooxygenase synthesize, respectively, the proinflammatory Series 2 prostaglandins and leukotriennes from the omega-6 fatty acid, arachidonic acid. Resveratrol irreversibly inactivates COX-1, the constitutive cyclooxygenase, and suppresses activation of the COX-2 gene induced by several protein kinases, including PKCa and the MAPK Erk1, thus inhibiting COX-2 formation and lowering inflammation.34
IκB Kinase: Anti-inflammatory effects
B sequesters NFκB in the cytoplasm, preventing its translocation to the nucleus where it activates numerous pro-inflammatory genes. IκB Kinase catalyzes the disassociation reaction through which NFκB is released, a core event in inflammation. Resveratrol suppresses IκB Kinase phosphorylation of IκB.34
PKC family: Cancer-prevention
In gastric and prostate cancer cells, resveratrol inhibits isoforms of the Protein Kinase C family of enzymes, causing inhibition of cancer cell growth and induction of apoptosis.34
MAPK Family: Cardiovascular protection
The mitogen-activated protein kinase family includes the extracellular signal regulated kinases (Erk1/2) and the stress activated kinases JNK1/2 and p38. Activation of stress kinases—prevented by resveratrol—is implicated in cardiovascular disorders.34
AMPK: Improved glucose metabolism
AMPK phosphorylation—increased by resveratrol—has been shown to protect cells from glucotoxicity, improve insulin sensitivity, and stimulate glucose transport comparable to the anti-diabetic drug metformin.34
Ribonucleotide Reductase: Cancer prevention
Ribonucleotide reductase catalyses the reduction of ribonucleotides (e.g., ATP) into the corresponding deoxyribonucleotides (single units of DNA) and is the target of anticancer drugs, such as gemcitabine and hydroxyurea, which are structurally similar to resveratrol. Resveratrol has been shown to be an even more potent inhibitor of ribonucleotide reductase than hydroxyurea.34
Quinone Reductase 2: Improving Cellular Redox Capacity
Quinone reductases are cytosolic flavoproteins (proteins that contain flavin adenine dinucleotide (FAD), a derivative of riboflavin), which catalyze the reaction that recycles NADP(H) to NADP+, thus playing a role in maintaining healthy cellular redox potential. Quinone reductase 2 has recently been revealed to be a key upstream factor integral to the inhibition of NFκB. Resveratrol’s upregulation of QR2 is thought to be one mechanism via which resveratrol regulates NFκB.343536 And given our above discussion of the pivotal importance of NAD+ salvage for sirtuin activation, this is also another mechanism through which resveratrol boosts sirtuin activity.
Aromatase: Modulating Estrogen Synthesis
Aromatase, a cytochrome P450 (Phase I) enzyme, catalyzes the rate limiting reaction in estrogen biosynthesis. Resveratrol inhibits aromatase activity and mRNA expression in breast cancer cells. Since “the synergistic action of unopposed estrogen and leptin, compounded by increasing insulin, cortisol and xeno-oestrogen exposure directly initiate, promote and exacerbate obesity, type 2 diabetes, uterine overgrowth, prostatic enlargement, prostate cancer and breast cancer,” this action of resveratrol may be highly significant.3437
Beyond Resveratrol: Other Life-Extending Plant Compounds
A number of other naturally occurring dietary polyphenols, e.g. quercitin (in apples and onions), curcumin (in the curry spice turmeric), fisetin (in strawberries), and epigallocatechin gallate (in green tea), also have potent antioxidant and anti-inflammatory properties, which are now known to be due not merely to their antioxidant effects but their modulation of key pro-inflammatory pathways, such as NFκB and MAPK-dependent signaling. Each of these polyphenols has also been shown to be a SIRT1 activator or STAC (sirtuin activating compound).38
Back in 2003 – a long way back in this rapidly evolving area – a research team including Howitz and Sinclair39 showed that the deacetylation activity of SIRT1 could be enhanced by a number of polyphenols including: resveratrol (up to 13-fold), butein (8.5-fold; found in Toxicodendron vernicifluum, a.k.a. Japanese laquer tree), piceatannol (7.9-fold; a metabolite of resveratrol found in red wine), fisetin (6.6-fold) and quercetin (4.6-fold). All SIRT1 activators (resveratrol included) share a common mechanism of action: they bind to the same site as nicotinamide and lower the Michaelis constant (Kм) for the substrate and for NAD+ .9
Conclusion
This field is new—but the data accumulated is already substantive and points to a very bright future for longevity medicine given what we already know about the sirtuins: (1) Sirtuins act as sensors of the metabolic state of the cell and organism. (2) Sirtuin activity is controlled by the cellular NAD+:NADH ratio and is therefore responsive to changes in cellular metabolism.40 (3) Perhaps the key reason why CR results in activation of the sirtuins: CR lowers metabolism and oxidative stress, increasing availability of NAD+.26 (4) In addition to CR, sirtuin activity can be upregulated by resveratrol and a number of other xenohormetic plant compounds. We have entered a new era in which the key genes and intracellular pathways responsible for aging and longevity have been identified.41 Lifespan is tractable, and we now know enough to begin utilizing emerging knowledge to develop effective longevity-enhancing supplements and protocols.
Read Part I: Resveratrol, Niacin, Nicotinamide Riboside: Key Players in Activating Sirtuins to Mimic Calorie Restriction & Extend Lifespan
References
- Dali-Youcef N, Lagouge M, Froelich S, et al. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med. 2007;39(5):335-45. ↑
Abstract - Marfe G, Tafani M, Pucci B, et al. The effect of marathon on mRNA expression of anti-apoptotic and pro-apoptotic proteins and sirtuins family in male recreational long-distance runners. BMC Physiol. 2010 May 12;10:7. ↑
Abstract - Weiss T, Hergeth S, Zeissler U, et al. Histone H1 variant-specific lysine methylation by G9a/KMT1C and Glp1/KMT1D. Epigenetics Chromatin. 2010 Mar 24;3(1):7. ↑
Abstract - Happel N, Doenecke D. Histone H1 and its isoforms: contribution to chromatin structure and function. Gene. 2009 Feb 15;431(1-2):1-12. ↑
Abstract - Vijg J, Maslov AY, Suh Y. Aging: a sirtuin shake-up? . Cell. 2008 Nov 28;135(5):797-8. ↑
Abstract - Shore D. Telomeric chromatin: replicating and wrapping up chromosome ends. Curr Opin Genet Dev. 2001 Apr;11(2):189-98. ↑
Abstract - Sundaresan NR, Samant SA, et al. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 2008 Oct;28(20):6384-401. ↑
Abstract - Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010 Aug;1804(8):1626-1634. ↑
Abstract - Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253-95. ↑
Abstract - Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009 Apr;20(2):98-105. ↑
Abstract - Chaudhary N, Pfluger PT. Metabolic benefits from Sirt1 and Sirt1 activators. Curr Opin Clin Nutr Metab Care. 2009 Jul;12(4):431-7. ↑
Abstract - Sugden MC, Caton PW, Holness MJ. . PPAR control: it’s SIRTainly as easy as PGC. J Endocrinol. 2010 Feb;204(2):93-104. Epub 2009 Sep 21. ↑
Abstract - Bordone L, Motta MC, Picard F, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006 Feb;4(2):e31. Epub 2005 Dec 27. ↑
Abstract - Li X, Zhang S, Blander G, et al. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007 Oct 12;28(1):91-106. ↑
Abstract - Wang GL, Fu YC, Xu WC, et al. Resveratrol inhibits the expression of SREBP1 in cell model of steatosis via Sirt1-FOXO1 signaling pathway. Biochem Biophys Res Commun. 2009 Mar 13;380(3):644-9. Epub 2009 Jan 31. ↑
Abstract - Chung S, Yao H, Caito S, et al. CROSS; Lee IH, Cao L, Mostoslavsky R, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008 Mar 4;105(9):3374-9. Epub 2008 Feb 22. ↑
Abstract - Chung S, Yao H, Caito S, et al. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch Biochem Biophys. 2010 May 5. [Epub ahead of print] . ↑
Abstract - Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008 Jun;20(3):303-9. Epub 2008 May 28. ↑
Abstract - Salminen A, Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009 May;15(5):217-24. Epub 2009 Apr 18. ↑
Abstract - Salminen A, Kaarniranta K. SIRT1: regulation of longevity via autophagy. Cell Signal. 2009 Sep;21(9):1356-60. Epub 2009 Feb 26. ↑
Abstract - Lim CS. SIRT1: tumor promoter or tumor suppressor? . Med Hypotheses. 2006;67(2):341-4. Epub 2006 Mar 20. ↑
Abstract - Singh S, Johnson J, Chellappan S. Small molecule regulators of Rb-E2F pathway as modulators of transcription. Biochim Biophys Acta. 2010 Jul 14. [Epub ahead of print] . ↑
Abstract - Wang C, Chen L, Hou X, et al. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006 Sep;8(9):1025-31. Epub 2006 Aug 6. ↑
Abstract - Yuan J, Minter-Dykhouse K, Lou Z. A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. J Cell Biol. 2009 Apr 20;185(2):203-11. Epub 2009 Apr 13. ↑
Abstract - Yi J, Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta. 2010 Aug;1804(8):1684-1689. ↑
Abstract - Vijg J, Maslov AY, Suh Y. Aging: a sirtuin shake-up? . Cell. 2008 Nov 28;135(5):797-8. ↑
Abstract - Stiewe T, Pützer BM. Role of p73 in malignancy: tumor suppressor or oncogene? . Cell Death Differ. 2002 Mar;9(3):237-45. ↑
Abstract - Marabese M, Vikhanskaya F, Broggini M. p73: a chiaroscuro gene in cancer. Eur J Cancer. 2007 Jun;43(9):1361-72. Epub 2007 Apr 10. ↑
Abstract - Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010 Apr;9(2):285-90. ↑
Abstract - Hirschey MD, Shimazu T, Goetzman E, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010 Mar 4;464(7285):121-5. ↑
Abstract - Kim HS, Patel K, Muldoon-Jacobs K, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010 Jan 19;17(1):41-52. ↑
Abstract - Schumacker PT. A tumor suppressor SIRTainty. Cancer Cell. 2010 Jan 19;17(1):5-6. ↑
Abstract - Sauve AA. Sirtuin chemical mechanisms. Biochim Biophys Acta. 2010 Aug;1804(8):1591-603. Epub 2010 Feb 2. ↑
Abstract - Pirola L, Fröjdö S. Resveratrol: one molecule, many targets. IUBMB Life. 2008 May;60(5):323-32. ↑
Abstract - Hsieh TC. Antiproliferative effects of resveratrol and the mediating role of resveratrol targeting protein NQO2 in androgen receptor-positive, hormone-non-responsive CWR22Rv1 cells. Anticancer Res. 2009 Aug;29(8):3011-7. ↑
Abstract - Hsieh TC, Wu JM. Resveratrol: Biological and pharmaceutical properties as anticancer molecule. Biofactors. 2010 Jul 7. [Epub ahead of print] . ↑
Abstract - Williams GP. The role of oestrogen in the pathogenesis of obesity, type 2 diabetes, breast cancer and prostate disease. Eur J Cancer Prev. 2010 Jul;19(4):256-71. ↑
Abstract - Allard JS, Perez E, Zou S, et al. Dietary activators of Sirt1. Mol Cell Endocrinol. 2009 Feb 5;299(1):58-63. ↑
Abstract - Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003 Sep 11;425(6954):191-6. Epub 2003 Aug 24. ↑
Abstract - Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006 Jul 28;126(2):257-68. ↑
Abstract - Morris BJ. A forkhead in the road to longevity: the molecular basis of lifespan becomes clearer. J Hypertens. 2005 Jul;23(7):1285-309. ↑
Abstract

Comments are closed.