Part I: Delaying the Mitochondrial Decay of Aging
Introduction
Mitochondrial decay resulting from oxidative damage is known to accumulate with age and is universally recognized as a major contributing factor to the whole range of functional decline and tissue deterioration associated with aging.1 2 3 4 5 6 7 Age-associated changes in mitochondria are characterized by increased generation of oxidants during oxidative phosphorylation and a decline in energy production, due in part to impaired enzyme activity and also to a decrease in cardiolipin, a phospholipid concentrated in the inner mitochondrial membrane that is essential for the function of key enzymes in the electron transport chain (see Glossary for more complete definition of italicized terms).8
Simplified Structure of a Mitochondrion
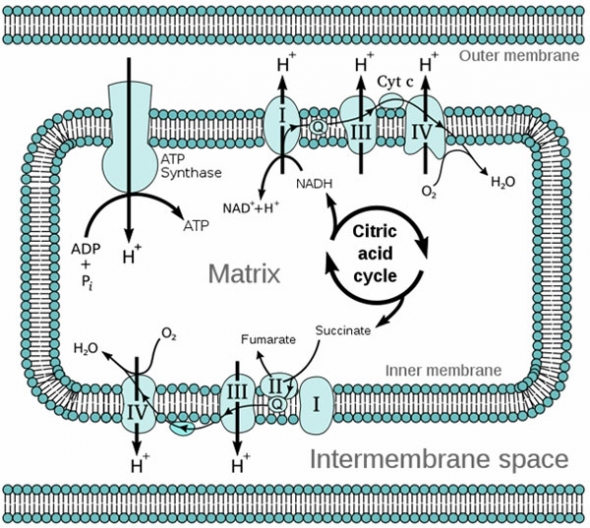
During the phase of ATP production in which oxidative phosphorylation takes place within the mitochondria, electrons from NADH or FADH2 are transferred through the electron transport chain (Complexes I through IV), with the end result that ATP is generated and molecular oxygen (O2) is reduced to water. However, in this process, even in youth, ~ 2% of the electrons escape the electron transport chain (ETC) and reduce O2 to the highly reactive oxidant species, superoxide radical (O2−) and hydrogen peroxide (H2O2). This basal leakage of oxidants from the ETC not only appears to be unavoidable, but increases with age, rendering mitochondria the main endogenous center of superoxide radical formation.8
A side effect of being the primary source of these toxic oxidants is that mitochondria become their immediate targets. Mitochondria’s proximity to the oxidants they produce (O2− and H2O2), combined with their intricate structure, and the age-associated decline in antioxidant capacity (whose underlying mechanisms are discussed in Parts II and III of this review), render these organelles especially vulnerable to oxidative damage. In addition, mitochondria lack catalase, which catalyzes the decomposition of H2O2 to water and oxygen; the ability to synthesize glutathione (GSH), the body’s premier intracellular antioxidant; the ability to transport glutathione disulfide (GSSG), the oxidized form of GSH, out of the matrix; and chelators for heavy metals – all of which serve as key mechanisms through which cells decrease oxidant production.8
Tuning Up Mitochondrial Function with Acetyl L-Carnitine and A-Lipoic Acid
For more than thirty years, the legendary Bruce Ames, PhD, whose more than 450 scientific publications have resulted in his being among the few hundred most-cited scientists in any field—he certainly qualifies for, and has, his own entry on Wikipedia9—has been conducting research focused on tuning up mitochondrial metabolism to optimize health and longevity.
Nutrients Required for Mitochondrial Energy Production22 23
Niacin (vitamin B3): The coenzyme nicotinamide adenine dinucleotide (NAD+) is derived from nicotinic acid (vitamin B3) and is required for reactions in all three phases of energy production: glycolysis, the Krebs cycle, and Complex I of the electron transport chain (ETC).
Biotin: also a B vitamin and required for heme biosynthesis. Heme is the major functional form of iron and is synthesized in the mitochondria.
Pantothenic acid (vitamin B5): Acetyl coA, the product of glycolysis that becomes the initial compound in the Krebs cycle, is synthesized from B5. B5 is also needed for heme synthesis.
Pyridoxine (vitamin B6): required for heme biosynthesis.
Riboflavin: This B vitamin is integral to succinate dehydrogenase (aka succinate-coenzyme Q reductase or Complex II), the only enzyme that participates in both the citric acid cycle and ETC. Riboflavin is also necessary for the activity of NADH dehydrogenase (aka Complex I of the ETC).
Ubiquinone (CoQ10): The carrier of electrons between Complexes I and II and to Complex III in the ETC.
Magnesium: Required by the Krebs cycle enzyme, isocitrate dehydrogenase.
Manganese: Because the primary source of oxidative stress in the cell is mitochondrial production of superoxide radical (O2−), the enzyme manganese superoxide dismutase (MnSOD), the only known scavenger of superoxide anion in the mitochondrial matrix, plays a critical role as the first line of defense in protecting the mitochondria from oxidative damage.24
Iron: An integral component of all four complexes of the ETC. NADH dehydrogenase (Complex I) contains 8 iron-sulfur clusters. Succinate dehydrogenase, contains 3 iron-sulfur clusters and a heme group. Cytochrome c, which transfers electrons between Complexes I and III, is a heme protein. Complex III, coenzyme Q:cytochrome c-oxidoreductase, which is sometimes called the cytochrome bc1 complex, is made up of three subunits, 2 of which contain heme and one of which has an iron-sulfur cluster. The enzyme cytochrome c oxidase or Complex IV, the last enzyme complex in the ETC, contains 2 hemes. Heme is the major functional form of iron and is synthesized in the mitochondria. Heme biosynthesis requires vitamin B6, riboflavin, biotin, pantothenic acid, lipoic acid, and the minerals zinc, iron, and copper.
Cysteine: Cysteine, an amino acid, provides the sulfur component of iron-sulfur clusters is therefore also required by all four complexes of the ETC.
Copper: Heme biosynthesis requires copper. Complex IV, the last enzyme complex in the ETC, contains 2 copper centers.
Zinc: required for heme biosynthesis.
D-ribose: a monosaccharide, is ubiquitous throughout the body as a constituent of RNA. Once phosphorylated, D-ribose also serves as a subunit of ATP and NADH. D-ribose has been shown to benefit patients with chronic fatigue syndrome and fibromyalgia.25 Since the heart’s ability to resynthesize ATP is limited by its supply of D-ribose, a necessary component of the adenine nucleotide structure, D-ribose has also been given as an ATP substrate supplement for the ischemic or hypoxic heart in which nucleotides (ATP, ADP, and AMP) are degraded and lost, and has been shown to increase tolerance to myocardial ischemia, and in patients with stable coronary artery disease, to improve time to exercise-induced angina.26 27 28
In recent studies, Ames and his team at the University of California, Berkeley, have made significant progress, reversing indicators of age-associated mitochondrial dysfunction in old rats by feeding them high doses of two crucial mitochondrial metabolites, acetyl L-carnitine and α-lipoic acid. Specifically, in old compared to young rats, mitochondrial membrane potential, cardiolipin level, respiratory control ratio, and cellular O2 uptake are lower, while levels of oxidants, and mutagenic aldehydes from lipid peroxidation are higher. Yet when old rats are given a diet containing high levels of acetyl-L-carnitine and α-lipoic acid, within just a few weeks, these signs of mitochondrial decay are reversed, restoring their mitochondrial function to a much more youthful level.10 11 12 13 14
While their mechanisms of action differ, acetyl L-carnitine and α-lipoic acid complement one another, in some cases synergistically.
Acetyl L-carnitine has been described as a conditionally essential nutrient for humans. In addition to glycolysis, acetyl-CoA is produced from the oxidation of fatty acids by acyl-CoA synthetase enzymes in the outer mitochondrial membrane, and then transported into the inner mitochondrial matrix by acetyl L-carnitine for β oxidation and ATP production. Acetyl L-carnitine also facilitates removal from the mitochondria of the excess short- and medium-chain fatty acids that accumulate during fat metabolism. Acetyl-L-carnitine is the preferred form of the nutrient in aging or conditions of disease since it is better absorbed and more efficiently crosses the blood–brain barrier compared to L-carnitine.15
Feeding old rats an acetyl-L-carnitine-supplemented diet restores tissue levels of free and acyl carnitines to those found in plasma and brain tissues of younger animals and, when combined with α-lipoic acid, significantly increases carnitine acetyltransferase (CAT) activity. More than 70% of CAT, an enzyme present in all mammalian tissues, is located in the mitochondrial matrix, where it catalyses the reversible conversion of acetyl-CoA and carnitine to acetyl carnitine and CoA. Thus CAT regenerates CoA, which allows peroxisomal β-oxidation to proceed, and facilitates transport of acetyl moieties to the mitochondria for oxidation. (β-oxidation of fatty acids generates acetyl-CoA, the entry molecule for the Krebs or citric acid cycle.)13
Alpha lipoic acid is a cofactor for pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, two key enzymes involved in the Krebs cycle. Not only does α-lipoic acid function as a mitochondrial coenzyme, but it also is reduced in the mitochondria to a powerful antioxidant, dihydrolipoic acid, which recycles other antioxidants, including vitamins C and E.13
In addition, α-lipoic acid is a potent inducer of the ~200 Phase II enzymes with antioxidant activity, including those required for the synthesis of GSH, the body’s most important intracellular antioxidant.
Thus supplementation with α-lipoic acid also increases intracellular GSH levels, which is critical for neuronal function. As a result, in aged rodents, α-lipoic acid supplementation restores long-term potentiation, a synaptic analogue of learning and memory, and partially restores ambulatory activity and memory lost during aging.13 16 17 18
As noted above, when old rats receive high levels of both acetyl L-carnitine and α-lipoic acid, within several weeks, the combination greatly improves mitochondrial function, resulting in restoration of ambulatory activity and cognition (as assayed with the Skinner box and Morris water maze), heart, and immune function to levels seen in young rats.
The KM Concept: Key to Mitochondrial Decay?
Ames’ rationale for the mechanism behind the rejuvenating effects of acetyl-L-carnitine and α-lipoic acid is that, with age, increased oxidative damage to key proteins, causes deformation in their structure, resulting in an increased Michaelis constant/(KM) or decreased binding affinity for a co-enzyme (i.e., the nutrient co-factor for the enzyme), and thus a decrease in enzyme function.19
Ames’ research has demonstrated that this age-associated effect on enzyme-binding affinity can be mimicked by exposing enzymes to malondialdehyde, a lipid-peroxidation product, levels of which also increase with age. Increasing availability of the substrate acetyl L-carnitine along with α-lipoic acid, a mitochondrial antioxidant, restores the velocity of the reaction (KM) for the enzyme acetyl-L-carnitine transferase and thus improves mitochondrial function.8
Common Nutrient Deficiencies Accelerate Mitochondrial Decay
Ames also targets iron and vitamin B6 as two common nutrient deficiencies that accelerate mitochondrial decay.20 Heme biosynthesis occurs predominantly in the mitochondria. Iron insufficiency disrupts heme synthesis resulting in loss of Complex IV and, as a result, significantly increased release of oxidants. Iron deficiency is fairly common among menstruating women in the U.S., 25% of whom ingest ≤50% of the DRI. Vitamin B6 deficiency, also not uncommon (10% of Americans ingest ≤50% of the DRI), can also cause heme deficiency since heme biosynthesis requires B6. A further consideration is that menstruating women may be using oral contraceptives, which have repeatedly been found to be associated with significantly lowered levels of vitamins B6 and B12.21 Ames notes that insufficiencies of iron or B6 are likely to result in accelerated aging and neural decay.
Conclusion
While it has commonly been thought that Americans’ intake of essential micronutrients is adequate, evidence indicates that damage occurs at levels higher than those that cause acute deficiency disease. In addition, as many as one-third of all single nucleotide polymorphisms (SNPs) in a gene result in the corresponding enzyme having an increased KM (decreased binding affinity) for its coenzyme, and therefore a lower rate of reaction. Given that some of these SNPs (to be discussed in Part II of this review: The Methylation Transsulfuration Connection to Mitochondrial Function) are found in ~30-40% of the population,29 it is obvious that higher than DRI levels are necessary for optimal function in significant numbers of people.
Dr. Ames’ KM concept applies both to aging-associated mitochondrial dysfunction, as discussed above in regards to acetyl-L-carnitine and lipoic acid, and to inborn (genetic) weaknesses in metabolism. In both cases, the functional capacity of defective enzymes can be ameliorated by administration of high doses of the corresponding cofactors, which raises coenzyme levels and at least partially restores enzymatic activity and mitochondrial function.
However, a protocol focused on restoration of KM, while certainly helpful in delaying mitochondrial decay, does not address a more fundamental issue – why does human physiology shift from a homeostasic state that repairs and balances itself to one that allows decay to accumulate? A new theory concerning what researcher Wulf Dröge has called “the first cause of death” may provide insight into a vicious cycle responsible for the shift from a state of youthful homeostatic repair to a homeostatic state that promotes mitochondrial decay. This will be the topic of Part III of this review: Reversing the Age-related Metabolic Shift towards Mitochondrial Decay.
Citric Acid (Krebs) Cycle
Image Source: http://en.wikipedia.org/wiki/File:Citric_acid_cycle_with_aconitate_2.svg
Mitochondrial Oxidative Phosphorylation (Electron Transport Chain)
Glossary
α-ketoglutarate dehydrogenase (aka xoglutarate dehydrogenase)—enzyme involved in the citric acid or Krebs cycle in which it catalyzes the reaction: α-ketoglutarate + NAD+ + CoA → Succinyl CoA + CO2 + NADH
β oxidation—the process by which fatty acids, in the form of Acyl-CoA molecules, are broken down in mitochondria and/or in peroxisomes to generate Acetyl-CoA, the entry molecule for the Krebs cycle.
betaine—in chemistry, any neutral compound with both a positively- and a negatively-charged functional group, so that it carries a total net charge of 0, while carrying formal positive and negative charges, also called a zwitterion. In biological systems, betaines, which are polar and water-soluble, permit water to remain in cells, thus serving as intracellular protectors against osmotic stress. Betaines also serve as methyl donors; trimethylglycine is a betaine.
cardiolipin—an important component accounting for ~20% of the total lipid content of the inner mitochondrial membrane, cardiolipin (so named since it was first identified in beef heart in the 1940s) is essential for the optimal function of numerous enzymes involved in mitochondrial energy production including cytochrome bc1 (Complex III) and cytochrome c oxidase (Complex IV) in the electron transport chain. Decreased cardiolipin synthesis is thought to be associated with mitochondrial dysfunction in Parkinson’s disease, non-alcoholic fatty liver disease, heart failure and diabetes.30 31 32 33 Increased mitochondrial ROS production promotes oxidation and depletion of cardiolipin, as well as inhibition of cytochrome c oxidase activity. Peroxidation of cardiolipin has been suggested to impair the barrier function of the inner membrane and facilitate the detachment of cytochrome c from the electron transport chain.24
catalase—a common enzyme found in virtually all organisms exposed to oxygen, catalase catalyzes the decomposition of hydrogen peroxide to water and oxygen. Catalase has one of the highest turnover numbers of all enzymes; one molecule of catalase can convert millions of molecules of hydrogen peroxide to water and oxygen per second.343
Glutathione—GSH is a tripeptide composed of cysteine, glycine, and glutamate that is synthesized de novo in all cells and serves as the major intracellular antioxidant and redox buffer.
heme—the major functional form of iron, synthesized in the mitochondria.36
pyruvate dehydrogenase—enzyme involved in transforming pyruvate from glycolysis into acetyl-CoA, which is then used in the citric acid or Krebs cycle. Thus, pyruvate dehydrogenase serves as a link from glycolysis to the citric acid or Krebs cycle, which is followed by oxidative phosphorylation in the electron transport chain of the mitochondria.
References
-
Harman D. Free radical theory of aging: history. EXS. 1992;62:1-10. ↑
Abstract -
Wei YH, Lu CY, Lee HC, et al. Oxidative damage and mutation to mitochondrial DNA and age-dependent decline of mitochondrial respiratory function. Ann N Y Acad Sci. 1998 Nov 20;854:155-70. ↑
Abstract -
Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta. 1998 Aug 10;1366(1-2):53-67. ↑
Abstract -
Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10771-8. ↑
Abstract -
Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998 Apr;78(2):547-81. ↑
Abstract -
Wei YH, Ma YS, Lee HC, Lee CF, Lu CY. Mitochondrial theory of aging matures—roles of mtDNA mutation and oxidative stress in human aging. Zhonghua Yi Xue Za Zhi (Taipei). 2001 May;64(5):259-70. ↑
Abstract -
Gruber J, Schaffer S, Halliwell B. The mitochondrial free radical theory of ageing—where do we stand?. Front Biosci. 2008 May 1;13:6554-79. ↑
Abstract -
Liu J, Atamna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci. 2002 Apr;959:133-66. ↑
Abstract -
Hagen TM, Yowe DL, Bartholomew JC, et al. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3064-9. ↑
Abstract -
Hagen TM, Liu J, Lykkesfeldt J, et al. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. roc Natl Acad Sci U S A. 2002 Feb 19;99(4):1870-5. ↑
Abstract -
Liu J, Head E, Gharib AM, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: Partial reversal by feeding acetyl-L-carnitine and/or R-α-lipoic acid. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2356-61. ↑
Abstract -
Liu J, Killilea D, Ames BN. Age-associated mitochondrial oxidative decay: Improvement of carnitine acetyltransferase substrate binding affinity and activity in brain by feeding old rats acetyl-L-carnitine and/or R-α-lipoic acid. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):1876-81. ↑
Abstract -
Hagen TM, Moreau R, Suh JH, et al. Mitochondrial decay in the aging rat heart: Evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann N Y Acad Sci. 2002 Apr;959:491-507. ↑
Abstract -
Ames BN. A role for supplements in optimizing health: the metabolic tune-up. Arch Biochem Biophys. 2004 Mar 1,423(1):227-34. PMID: 14989256, Ames BN. Mitochondrial decay, a major cause of aging, can be delayed. J Alzheimers Dis. 2004 Apr;6(2):117-21. ↑
Abstract -
Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997;22(1-2):359-78. ↑
Abstract -
Suh JH, Shenvi SV, Dixon BM, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci USA. 2004;101:3381-6. ↑
Abstract -
Suh JH, Wang H, Liu RM, Liu J, Hagen TM. (R)-alpha-lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: Evidence for increased cysteine requirement for GSH synthesis. Arch. Biochem. Biophys. 2004;423:126-35. ↑
Abstract -
Ames BN, Elson-Schwab I, Silver EA. High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased K(m)): relevance to genetic disease and polymorphisms. Am J Clin Nutr. 2002 Apr;75(4):616-58. ↑
Abstract -
Ames BN. The metabolic tune-up: metabolic harmony and disease prevention. J Nutr. 2003 May;133(5 Suppl 1):1544S-8S. ↑
Abstract -
Lussana F, Zighetti ML, Bucciarelli P, Cugno M, Cattaneo M. Blood levels of homocysteine, folate, vitamin B6 and B12 in women using oral contraceptives compared to non-users. Thromb Res. 2003;112(1-2):37-41. ↑
Abstract -
Atamma H. Heme, iron, and the mitochondrial decay of aging. Ageing Res Rev. 2004 Jul;3(3):303-18. ↑
Abstract -
Voet D, Voet J, Pratt C. Chapters 16, Glycogen Metabolism, Chapter 17 Citric Acid Cycle, Chapter 18 Electron Transport and Oxidative Phosphorylation. Fundamentals of Biochemistry. 3rd Edition, John Wiley & Sons, Inc.: NY, 2008, p.486-7,567-590, 596-637. ↑
-
Lee HC, Wei YH. Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med (Maywood). 2007 May;232(5):592-606. ↑
Abstract -
Teitelbaum JE, Johnson C, St Cyr J. The use of D-ribose in chronic fatigue syndrome and fibromyalgia: a pilot study. J Altern Complement Med. 2006 Nov;12(9):857-62. ↑
Abstract -
Pauly DF, Pepine CJ. D-Ribose as a supplement for cardiac energy metabolism. J Cardiovasc Pharmacol Ther. 2000 Oct;5(4):249-58. ↑
Abstract -
Sinatra ST. Metabolic cardiology: the missing link in cardiovascular disease. Altern Ther Health Med. 2009 Mar-Apr;15(2):48-50. ↑
Abstract -
Sinatra ST. Metabolic cardiology: an integrative strategy in the treatment of congestive heart failure. Altern Ther Health Med. 2009 May-Jun;15(3):44-52. ↑
Abstract -
Ogino S , Wilson RB. Genotype and haplotype distributions of MTHFR677C>T and 1298A>C single nucleotide polymorphisms: a meta-analysis. J Hum Genet. 2003;48:1-7. ↑
Abstract -
Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinsons disease. Science. 2003 Oct 31;302(5646):819-22. ↑
Abstract -
Petrosillo G, Portincasa P, Grattagliano I, et al. Mitochondrial dysfunction in rat with nonalcoholic fatty liver Involvement of complex I, reactive oxygen species and cardiolipin. Biochim Biophys Acta. 2007 Oct;1767(10):1260-7. ↑
Abstract -
Sparagna GC, Chicco AJ, Murphy RC, et al. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007 Jul;48(7):1559-70. ↑
Abstract -
Han X, Yang J, Yang K, et al. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry. 2007 May 29;46(21):6417-28. ↑
Abstract -
Zamocky M, Furtmüller PG, Obinger C. Evolution of catalases from bacteria to humans. Antioxid Redox Signal. 2008 Sep;10(9):1527-48. ↑
Abstract -
Goodsell DS. Catalase: Molecule of the Month. RCSB Protein Data Bank. 8-11-09. ↑
Abstract -
Atamma H. Heme, iron, and the mitochondrial decay of aging. Ageing Res Rev. 2004 Jul;3(3):303-18. ↑
Abstract



Comments are closed.